Thermochemical Equations
Thermochemical Equations
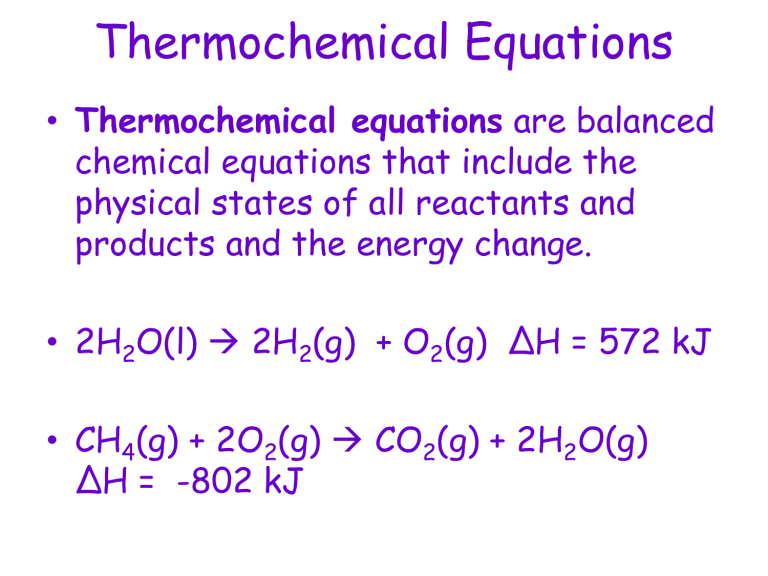
• Thermochemical equations are balanced chemical equations that include the physical states of all reactants and products and the energy change.
• 2H
2
O(l) 2H
2
(g) + O
2
(g) ΔH = 572 kJ
• CH
4
(g) + 2O
2
(g) CO
2
ΔH = -802 kJ
(g) + 2H
2
O(g)
Phase Changes
• Occurs when energy is added or removed from a system and the substance can go from one physical phase to another
Enthalpy of Combustion
• Enthalpy heat of combustion (ΔH comb the enthalpy change for the complete burning of one mole of the substance.
) is
-- carried out under standard conditions which are one atmospheric pressure (1 atm) and 298K (25 0 C)
- C
6
H
12
ΔH o comb
0
6
(s) + 6O
2
(g) 6CO
= -2808kJ
2
(g)+ 6H
2
O(l)
Substance
Sucrose
Octane
Glucose
Propane
Ethanol
Methane carbon
Enthalpy of Combustion
Formula
C
12
H
22
O
11
C
82
H
18
(l)
C
6
H
12
O
6
(s)
C
3
H
8
(g)
C
2
H
5
OH(l)
CH
4
(g)
C(s)
H comb
-5644
-5471
-2808
-2219
-1367
-891
-394
Phase Changes
• Occurs when energy is added or removed from a system and the substance can go from one physical phase to another
Changes of State
• Molar enthalpy (heat) of vaporization
(ΔH vap
) is the heat required to vaporize one mole of liquid.
-- think of water vaporizing from your skin after you take a hot shower. Your skin provides the heat needed to vaporize the water and as the water absorbs the heat you feel cool (shiver)
ΔH vap
= -ΔH cond
(condensation)
Changes of State
• Molar enthalpy (heat) of fusion (ΔH a solid substance.
fus
) is the heat required to melt one mole of
--think of ice in a drink. The drink cools as it provides the heat for the ice to melt
ΔH fus
= - ΔH solid
(solidification—freezing)
Substance
Water
Ethanol
Methanol
Ammonia
Changes of State
Formula
H
2
O
C
2
H
5
OH
CH
3
OH
NH
3
Hvap kJ/mol
40.7
38.6
35.2
23.3
Hfus kJ/mol
6.01
4.92
3.22
5.66
??What do you notice about the magnitude of the molar enthalpy of vaporization versus the molar enthalpy of fusion?
The molar enthalpy of vaporization for a substance is much larger than the molar enthalpy of fusion for the same substance. It takes much more energy to change a substance from a liquid to a gas than it does to change a solid to a liquid.
Endothermic Phase Changes
Melting
• The energy absorbed to melt a solid is not used to raise the temperature of that solid
• The energy instead disrupts the bonds holding the solid’s molecules together and cause the molecules to move into the liquid phase
Endothermic Phase Changes
• The amount of energy required to melt one mole of a solid depends on the strength of the forces that hold the solid together
• The melting point of a crystalline solid is the temperature at which the forces holding its crystal lattice together are broken and it becomes a liquid
Endothermic Phase Changes
Vaporization
• Particle that escape from the liquid enter the gas phase and those liquids at room temperature the gas phase is called vapor
• Vaporization is the process by which a liquid changes into a gas or vapor
• Once the solid becomes a liquid then and only then does the temperature of the substance begin to increase
Endothermic Phase Changes
• When vaporization takes place only at the surface of the liquid it is called evaporation
• Evaporation is the method by which the human body maintains and controls its temperature
Endothermic Phase Changes
Sublimation
• Is the process by which a solid changes directly to a gas without first becoming a liquid
• Dry ice (CO
2
) and snow are the most common examples
Endothermic Phase Changes
• If ice cubes are left in the freezer for extended periods of time, they will eventually sublime and become smaller
– This process is also helpful in freeze drying foods for hikers and astronauts
Exothermic Phase Changes
Condensation
• When a vapor molecule loses energy its velocity is reduced therefore colliding more with other molecules to form a liquid
• Condensation is the process by which a gas or vapor becomes a liquid and it is the reverse action of vaporization
Exothermic Phase Changes
Deposition
• Is the process by which a substance changes from a gas or vapor to a solid without first becoming a liquid
• It is the reverse action of sublimation
• The formation of snow crystals high up in the atmosphere is an example
Exothermic Phase Changes
Freezing Point
• Is the temperature at which a liquid is converted into a crystalline solid
• The same temperature as the melting point of a given substance
Phase Change Graph
Phase Change Graph
• Graph shows the energy required to go from one phase to the other
• Where the graph inclines , potential energy is at its greatest and temperature is increasing
• Where the graph plateaus ( flat region ) kinetic energy is at its greatest but the temperature remains constant
Phase Diagrams
• A phase diagram is a graph of pressure versus temperature that shows in which phase a substance exists under different conditions of temperature and pressure
Phase Diagrams
The triple point is the point on a phase diagram that represents the temperature and pressure at which three phases of a substance can coexist
The critical point is the point that indicates critical pressure and temperature above which water cannot exist as a liquid
Phase Diagrams
• Different for each substance because of the different boiling/freezing points
• Play video from Khan
Academy
Play