ch12 _and_17notes_20..
advertisement

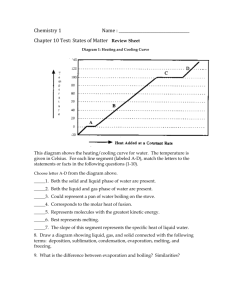
Ch. 12 Liquids and Solids, Liquids Fluid: ______________________________________ High Density _______________________________ Relative Incompressibility ___________________________________ Ability to diffuse _______________________________ Surface Tension ___________________________________ Capilary Action ___________________________________ Evaporation ___________________________________ Boiling ___________________________________ Solids pp 370 Crystals Ionic ___________________________________ Covalent Network ___________________________________ Metallic ___________________________________ Covalent ___________________________________ Change of State Equilibrium ___________________________________ Equilibrium vapor Pressure of a Liquid (Fig. 12-12 p. 377) Volatile liquids ___________________________________ Boiling and C _______________ occur when ____________________ Molar Heat of Vaporization (Hv =40.7 kJ/mol) Freezing and Melting ___________________________________ Molar Heat of Fusion (Hf =334 J/g) Add Heat from the system: Remove Heat from the system: Freezing is an process Melting is an process Q = m Cp T M = ______________Cp = ______________ T _______________ Use absolute value for T Use correct sign of Q by Exo/Endo determination: q = ΔH (mass/molar mass) for evaporation or fusion http://www.chemteam.info/Thermochem/Time-Temperature-Graph.html Be careful using g or mol q = ΔHvap (mass/molar mass) = 40.7 kJ / mol evaporation or condensation Example #1 49.5 g of H2O is being completely boiled at its boiling point of 100 °C. How many kJ is required? Ans. 111.9 kJ Example #2: 80.1 g of H2O exists as a gas at 100 °C. How many kJ must be removed to turn the water into liquid at 100 °C. Solution: note that the water is being condensed. The molar heat of vaporization value is used at the solid-liquid phase change, REGARDLESS of the direction (boiling or condensing). Example #3: calculate the heat of vaporization for water in J/g Ans. 2260J/g q = ΔHfus (mass/molar mass) = 6.02 kJ/mol fusion solidification or melting Example #1: 31.5 g of H2O is being melted at its melting point of 0 °C. How many kJ is required? Ans. 10.5 kJ Example #2: 53.1 g of H2O exists as a liquid at 0 °C. How many kJ must be removed to turn the water into a solid at 0 °C Solution: note that the water is being frozen and that there is NO temperature change. The molar heat of fusion value is used at the solid-liquid phase change, REGARDLESS of the direction (melting or freezing). Example #3: calculate the heat of fusion for water in J/g Ans. 334J/g Other sections of graphs have different Cp values Cp for solid water = 2.06 J/ g °C Cp for liquid water = Cp = 4.184 J/ g °C Cp for solid water = 1.87J/ g °C Problem #1: Calculate the heat necessary to raise 27.0 g of water from 10.0 °C to 90.0 °C The important factor about this problem is that ONLY temperature change is involved. Therefore, the equation to use is: q = (mass) (Δt) (Cp) Four Equations Needed Problem #1: 33.3 grams of ice at 0.00 °C has heat added to it until steam at 150.0 °C results. Calculate the total energy expended. (Hint: melt, raise, boil, raise.) and Ch.17 Thermochemistry Define the following terms: Thermochemistry ______________________________ Heat _____________________________ Temperature _______________________ Calorimeter _________________________ Specific heat_____________________________ Specific heat of Al ____________________________ As the temperature of 50.0 grams of Al changes from 20.0 C to 70.0 C, how much energy is required? ___________________ Specific heat of Pb ___________________ As the temperature of 50.0 grams of Pb changes from 20.0 C to 70.0 C, how much energy is required? ___________________ How much heat can be gained by Pb when Pb changes Temperature from 300 K to 350 K Write the thermochemical reaction for the synthesis of water (p.515) ___________________ What is a thermochemical equation? _________________________ What is enthalpy? ____________________________ Draw the exothermic reaction pathway – label everything from Fig. 17-2 Draw the endothermic reaction pathway – label everything from Fig. 17-3 What is the molar heat of formation? ________________________________________ Skip the rest of this unit 17-1 Section 17-2 Driving Force of Reactions What is the relationship between enthalpy and reaction tendency? _________________________________________________________________________________ What is the relationship between entropy and reaction tendency? _________________________________________________________________________________ What is entropy? ____________________________________________________ What is free energy? ____________________________ Write the reaction for free energy ________________________________________ If free energy has a positive value, is the reaction spontaneous? If free energy has a negative value, is the reaction spontaneous? If a reaction occurs at 25 C, and releases 160 kJ/mol of energy and has an entropy value of positive 50 J/mol K, what is the free energy of the reaction? Is it spontaneous? Section 17-3 Reaction Process What is a reaction mechanism? ____________________________________________________ What is collision theory? ___________________________________________ What is activation energy? _____________________________________________ Draw Figure 17-10, label all parts well, p. 534 Do sample problem 17-5 p.536, label well, then do the practice problem on p. 537 Section 17-4 Reaction Rate Define reaction rate _________________________________________________ Define chemical kinetics ________________________________ Rate Influencing factors List: Draw Figure 17-15 with reaction with no catalyst and the reaction with one catalyst, label all parts well. Skip Rate Laws