Enthalpy: Changes of State
advertisement

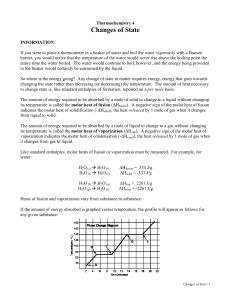
Chemistry 12 KE= energy of motion PE= stored energy T = measure of average KE of particles of system (or surroundings) A change in matter without any change in chemical composition of the system Just like chemical reactions, changes of state involve P.E changes in the system *** Always involves energy changes, but not temperature changes. There is constant temperature during a phase change. Why? 1 This is not a change in kinetic energy But we know that energy is entering the system because the bonds that are holding the molecules together are being broken or altered. This increases PE of the molecules It requires 10-100 times more energy for a chemical reaction to take place than a phase change Why? Because a chemical reaction requires stronger ionic and covalent bonds to be broken, but a phase change only requires intermolecular bonds to be broken. In general, enthalpy changes btw liquid and gas are greater than btw liquid and solid. Why? 2 (s) to (l) (l) to (g) Fusion (melting) vaporization endo T endo T (g) to (l) condensation exo T (l) to (s) exo T (g) to (s) Solidifying (freezing) sublimation exo T (s) to (g) sublimation endo T Aka: Latent Heat (of Phase Change) You can find tables of these values listed in data booklet as well as pg. 647 Units: kJ/mol 3 ∆Hvap = - ∆Hcond ∆Hmelt = - ∆Hfreeze (or solidification) Freezing (solidification) and condensing releases heat to surroundings Vaporization and melting absorb heat from surroundings Same units Molar Heat of Fusion (melting) = energy that must be absorbed in order to convert one mole of solid to liquid at its melting point (∆H melt ) Molar Heat of Freezing (solidifying) = energy that must be removed in order to convert one mole of liquid to solid at its freezing point (- ∆H freeze) Molar Heat of Vaporization = energy that must be absorbed in order to convert one mole of liquid to gas at its boiling point (∆H vap ) Molar Heat of Condensation = energy that must be removed in order to convert one mole of gas to liquid at its condensation point (- ∆H cond) 4 H2O (s) + 6.02 kJ H2O (l) (thermochemical) or H2O (s) H2O (l) ∆H0fus = 6.02 kJ Calculate how much energy is needed to convert 60g of ice at 0 ° C to liquid water at 0 ° C? 60g x 1 mol H2O = 3.3 mol H2O 18.02 g q = n B ∆H0fus = (3.3 mol) (6.009 KJ) = 19.8 kJ Pg 648-649 # 24-26,28,29 5