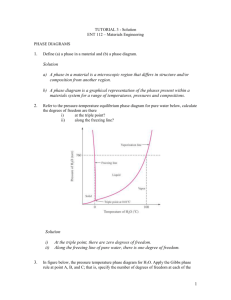
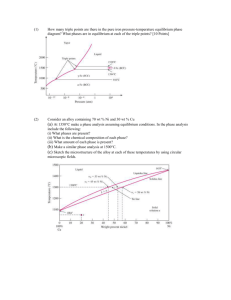
Thermal Equilibrium Diagrams: Alloys & Phase Changes
advertisement

Thermal Equilibrium Diagrams show the temperatures at which phase changes take place in alloys of different percentage composition. A Phase is a portion of a system that has uniform physical and chemical characteristics. Thermal Equilibrium Diagrams are made for information gained form Cooling Curves. When the temperature of a cooling molten metal alloy is plotted against time, a cooling curve is formed. Remember this only happens for pure metals. When cooling a solid pure metal, change from a solid to a liquid at constant temperature. This is called the Melting Point Temperature. The heat that is released in the transition from a solid to a liquid is called hidden or Latent Heat. LIQUID Solidification Starts Solidification Ends SOLID Latent heat is the quantity of heat energy absorbed or released when a substance changes its physical phase at constant temperature. The energy that goes in breaks the bond of the atoms. Pure Metal change directly LIQUID from Liquid to Solid at 1 temperature. SOLID 1500 Temperature 0C If a metal is 100% pure and contains no traces of other elements then some under cooling may occur before solidification begins. Under cooling is when the temperature drops below the liquid to solid temperature for a short period. Undercooling 1100 1000 Time Fall in temperature stops temporarily at the freezing point because of Latent heat Alloys change from Liquid to Solid through a pasty state over a temperature range. LIQUID Solidification Starts “PASTY” STATE Solidification Ends SOLID Pure Metal Alloy Over a Temperature Range 1Temperature A Solid Solution is when two metals are completely soluble in each other in both the liquid and solid states. When viewed under a microscope, a solid solution appears like a pure metal. Iron Carbon 100% Lead The given table shows the solidification temperatures for various alloys of Copper and Nickel. The melting point of Copper is 1083°C and Nickel is 1453°C. Using the graph paper supplied: (i) Draw the equilibrium diagram according to the given data. (ii) Label the diagram and describe the main features. (iii) For the alloy with 50% Nickel determine, from the diagram, the ratio of the phases at 1250°C. TEMPERATURE (°C) 1600 1400 1200 1000 800 600 400 200 NICKEL COPPER 0% 100% 20% 80% 40% 60% 60% 40% PRECENTAGE COMPOSITION 80% 20% 100% 0% 1400 TEMPERATURE (°C) 1200 1000 800 600 400 200 NICKEL 0 0% 10% 20% 30% 40% 50% 60% 70% COPPER PRECENTAGE COMPOSITION 80% 90% 100% Equilibrium diagrams are made from taking information for cooling of different percentage of an alloy and putting it on one graph. This is know as a TERMAL ANALYSIS 100% Copper 100% NICKLE 60% Copper 40% Nickel 40% Copper 60% Nickel TEMPERATURE (°C) 1600 Liquid Phase Liquidus Line 1400 1200 1000 800 Solid Phase 600 Phases are defined as regions that differ from one another on an equilibrium diagram. 400 Phases defined by composition (not by state – solid, liquid, gas) 200 NICKEL COPPER 0% 100% 20% 80% 40% 60% 60% 40% PRECENTAGE COMPOSITION 80% 20% 100% 0% Liquid: the two metals are soluble in each other in the liquid state. Liquidus Line: the change from fully liquid to pasty state. Above the liquidus line, the alloy is liquid. This is the beginning of solidification. Liquid and Solid: the alloy is in a pasty form, there are two phases one Liquid and one Solid. Solidus Line: the change from pasty to solid. Below the solidus line, the alloy is cooling and solid. This is the end of solidification. Solid: alloy is in solid form. Equilibrium may be defined as a state of balance or stability. Diagrams indicates the phases the alloy is at different temperatures. Phases are defined as regions that differ from one another on an equilibrium diagram. Phases defined by composition. 27 Equilibrium refers to Balance existing in a system In Metallurgy, Equilibrium means, that the cooling of a metal or an alloy is so slow that all the changes that might take place get the chance to do so. To achieve equilibrium to take place enough time for complete diffusion is required. Metallurgy is the study of metals and their properties Diffusion is the processes by which a substance spread out through another substance. Atoms move in a solid metal in this manner. HIGH CONSTANTRATION LOW CONSTANTRATION Atom move form high concentration to low concentration How Many Phases Are There? PHOTOS A SINGLE PHASE TWO-PHASES A Phase is a portion of a system that has uniform physical and chemical characteristics. Phases are defined as regions that differ from one another on an equilibrium diagram. Phases defined by composition. NOT BY STATE (solid, liquid , gas) Nickel Copper Copper Nickel Copper Nickel iii)For the alloy with 50% Nickel determine, from the diagram, the ratio of the phases at 1250°C. Tie Line 1400 1250°C TEMPERATURE (°C) 1200 1000 800 600 400 200 NICKEL 0 0% 10% 20% 26% 30% 40% 50% 60% 66%70% COPPER PRECENTAGE COMPOSITION 80% 90% 100% A 26% Ratio of phases at 1250℃ for 50% Nickel The ratio of Solid to Liquid is: B C 66% 1400 1250°C TEMPERATURE (°C) 1200 1000 800 600 400 200 NICKEL COPPER 0 0% 10% 20% 26% 30% 40% 50% 60% 66%70% 35% PRECENTAGE COMPOSITION Ratio of phases at 1250°C for 35% Nickel 80% 90% 100% 1400 1250°C TEMPERATURE (°C) 1200 1000 800 600 400 200 NICKEL 0 0% 10% 20% 26% 30% 40% 50% 60% 66%70% COPPER PRECENTAGE COMPOSITION Ratio of phases at 1250ºC for 60% Nickel 80% 90% 100% In a eutectic alloy the two metals are completely soluble in the liquid phase but are insoluble in the solid phase. The given table shows the solidification temperatures for various alloys of Cadmium and Nickel. Using the graph paper supplied: i. Draw the equilibrium diagram according to the given data. ii. Label the diagram and describe the main features. TEMPERATURE (°C) 1600 1400 1200 1000 800 600 400 200 BISMUTH 0% CABMIUM 100% 20% 80% 40% 60% 60% 40% PRECENTAGE COMPOSITION 80% 20% 100% 0% TEMPERATURE (°C) PRECENTAGE COMPOSITION 400 TEMPERATURE (°C) 350 300 250 200 150 100 50 0 BISMUTH 0% 10% 20% 30% 40% 50% 60% 70% CADMIUM PRECENTAGE COMPOSITION 80% 90% 100% 400 350 Liquidus Line Liquid Phase TEMPERATURE (°C) 300 Eutectic Point 250 200 Liquid Phase and Solid Phase Liquid Phase and Solid Phase 150 100 Solid Phases 50 0% BISMUTH CADMIMUM 100% 20% 80% 40% 60% 60% 40% PRECENTAGE COMPOSITION 80% 20% 100% 0% TEMPERATURE (°C) 400 350 Liquid Phase 300 Eutectic Point 250 200 Liquid and Solid Bismuth Liquid and Solid Cadmium 150 Solid Cadmium and Eutectic 100 BISMUTH CADMIUM Solid Bismuth and Eutectic 50 0% 100% 20% 80% 40% 60% 60% 40% 80% 20% PRECENTAGE COMPOSITION Solid Cadmium 100% 0% Green Line Eutectic Alloy Solid Bismuth Eutectic ∘ Cadmium Atoms ∙ Bismuth Atoms X 1 000 X 10 000 000 The point where the liquid alloy changes to solid without going through a liquid/solid state is called the eutectic point. Some metals in an alloy only partially dissolve in each other. Lead/Tin Alloy (Solder) is an example of this. The equilibrium diagram for this type of alloy is called a Partial Solubility thermal equilibrium diagram. It is a combination of the solid solution and eutectic diagrams and is a little more complex. Eutectic Alloys Solid Solution Alloys Solid Solution Alloys Partial Solubility Alloy 400 Liquidus Line Liquid and Solid TEMPERATURE (°C) 300 Liquid Phase Eutectic Point Liquid and Solid 200 100 Solid Phases TIN LEAD 0% 100% 20% 80% 40% 60% 60% 40% PRECENTAGE COMPOSITION 80% 20% 100% 0% 400 Liquidus Line Liquid and Solid Liquid Phases Solid Solution TEMPERATURE (°C) 300 Liquid and Solid 200 Solid Solution Solidus Line SOLVUS LINES 100 Solid TIN LEAD 0% 100% 20% 80% 40% 60% 60% 40% PRECENTAGE COMPOSITION 80% 20% 100% 0% 400 α Solid and Liquid TEMPERATURE (°C) α Solid Solution Liquid Phases 300 β Solid and Liquid 200 β Solid Solution α and β Solid Solution 100 TIN LEAD 0% 100% 20% 80% 40% 60% 60% 40% PRECENTAGE COMPOSITION 80% 20% 100% 0% Tin Lead Copy the given lead-tin diagram into your answer book and answer all of the following: (i) Identify the lines labelled A, B and C; (ii) Explain what each line represents; (iii) For the alloy with 30% tin determine, from the diagram, the composition of the phases at 250°C; (iv) Indicate clearly on your diagram the eutectic point. Liquidus line Solidus line Solvus line A – Liquidus line. For the alloy system this line represents the boundary between the fully liquid state and the beginning of solidification. B – Solvus line. The transition line from one solid form to another solid form of an alloy is called the solvus line. This line indicates the maximum amount of tin which can be dissolved in the lead. C – Solidus line. The boundary line that determines the end of solidification. Below this line the alloy is completely solid. To determine composition of the phases A B A – Solid composition consisting of 8% tin and 92% lead. B – Liquid composition consisting of 43% tin and 57% lead. Eutectic Point TEMPERATURE (°C) 1600 Liquid Phase Liquidus Line 1400 1200 1000 800 Solid Phase 600 Phases are defined as regions that differ from one another on an equilibrium diagram. 400 Phases defined by composition (not by state – solid, liquid, gas) 200 NICKEL COPPER 0% 100% 20% 80% 40% 60% 60% 40% PRECENTAGE COMPOSITION 80% 20% 100% 0% 400 350 Liquidus Line Liquid Phase TEMPERATURE (°C) 300 Eutectic Point 250 200 Liquid Phase and Solid Phase Liquid Phase and Solid Phase 150 100 Solid Phases 50 0% BISMUTH CADMIMUM 100% 20% 80% 40% 60% 60% 40% PRECENTAGE COMPOSITION 80% 20% 100% 0% 400 α Solid and Liquid TEMPERATURE (°C) α Solid Solution Liquid Phases 300 β Solid and Liquid 200 β Solid Solution α and β Solid Solution 100 TIN LEAD 0% 100% 20% 80% 40% 60% 60% 40% PRECENTAGE COMPOSITION 80% 20% 100% 0% You should know that a pure metal has one clearly defined melting point. Mixtures tend to solidify over a temperature range, that is they start to solidify at one temperature and do not complete the process until they reach a lower point. A mix of 80% A with 20% B will solidify over a different range than a mix of 20% A with 80% B. A phase diagram allows us to present all this information in a single (sometime simple) diagram. Equilibrium may be defined as a state of balance or stability. Diagrams indicates the phases the alloy is at different temperatures. Phases are defined as regions that differ from one another on an equilibrium diagram. Phases defined by composition. 68