hw3 _2010_11 - Rose
advertisement

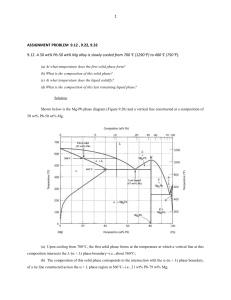
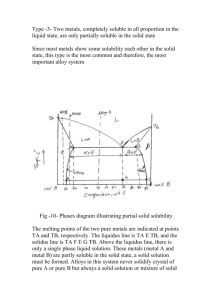
ME328 Materials Engineering Homework 3 The article “Energy Efficient Tempers for Aluminum Motorcycle Cylinder Blocks” describes the processing of an aluminum-silicon alloy. Without the hardness provided by the silicon, aluminum would not be sufficiently wear resistant for an engine block (unless combined with another material, e.g. a cast iron cylinder liner). The article uses vocabulary words like solidus, hypereutectic, primary that are useful to know. 1. 2. 3. 4. 5. The first sentence in the article refers to the “high-performance motorcycle” shown in figure 1. In what alternate reality is a 250 cc scooter viewed as “high-performance motorcycle”? (no answer required) The “block is ejected from the mold when its temperature is below solidus (approximately 380C)”. a) Define “solidus” b) State the solidus temperature for a 20% silicon alloy. (Note the included Al-Si phase diagram). The block is “made of a hypereutectic Al-Si alloy”. a) Define “eutectic” b) Define “hypereutectic alloy” c) Name the eutectic temperature for Al-Si. ______________ d) Name the eutectic composition for Al-Si. ______________ The hardness of the block is “determined by the volume fraction of primary and eutectic silicon phases”. a) Define primary phase b) Name the primary phase for a 25% Si alloy. _______________ c) Name the phases present in the eutectic grains of the 25% Si alloy. _________________ d) Determine the relative amount of primary phase in a 25% Si alloy that has been slow cooled from a liquid. The heat treatment of the 25% Si block includes “solution treatment at 490C” and “aging at 200C”. a) How many phases are present at 490C under equilibrium conditions? b) Name the phases present at 490C under equilibrium conditions. 6. The following link takes you the NIST site that shows Binary and Ternary Phase Diagrams for a number of combinations of elements. Please consider the Bi-Sn and Ag-Cu Systems http://www.metallurgy.nist.gov/phase/solder/solder.html For each System, name the Eutectic temperature, the Eutectic Composition, and the melting range of a 50-50 alloy. System Eutectic Temp Eutectic Comp Melting Range (% of each alloy) (temperature) Bi-Sn Ag-Cu 7. Use the Ag-Cu diagram to answer the following a) name the temperature that a 70%Ag-30%Cu alloy will be fully liquid Name the phase(s) present, the chemical compositions of each phase and the relative amounts of each phase for a 70%Ag-30%Cu alloy at b) 1100C c) 825C d) 200C 8. Go to http://www-g.eng.cam.ac.uk/mmg/teaching/ and select Iron-Carbon Phase Diagram from the Teach Yourself Phase Diagrams section. Go through the example to see how the eutectoid microstructure and the hypoeutectoid forms. a) name the eutectoid microstructure for iron carbon b) state the difference between eutectic and eutectoid c) What phases are present in the eutectoid microstructure d) What carbon composition would a hypo-eutectoid microstructure have? e) For a eutectoid composition steel alloy, name the phase(s) present at 800C. f) Describe the microstructure of a 1020 steel at room temperature in terms of the phases and micro constituents present (a sketch may be helpful). (extra fun but not extra credit) If you want to know more about how a phase diagram can be determined experimentally, the following web site is good. Note that the authors have focused this on 16-18 year olds. http://www.chemguide.co.uk/physical/phaseeqia/snpb.html