Materials Science Problems: Nucleation, Solidification
advertisement

Chapter 4 Problems
2.
4
Use equations 4.4 [ Gr r 3 Gv 4r 2 SL ] and 4.10 [ nr n0 exp Gr / kT ] to estimate
3
the number of crystal-like clusters in 1 mm3 of copper at its melting point for spherical clusters
containing (a) 10 atoms, (b) 60 atoms. What volume of liquid copper is likely to contain one
cluster of 100 atoms? The atomic volume of liquid copper is 1.610-29 m3, SL is 0.177 J/m2, k
= 1.3810-23 J/K, Tm = 1356 K.
4.
Calculate the homogeneous nucleation rate in liquid copper at undercoolings of 180, 200 and
220 K, using the following data: L = 1.88109 J/m3, Tm = 1356 K, SL = 0.177 J/m2, f0 = 1011 s-1,
C0 = 61028 atoms/m3, k = 1.3810-23 J/K.
6.
4
Show that Equation 4.16 [ Ghet r 3 Gv 4r 2 SL S ] follows from 4.15
3
( Ghet VS Gv ASL SL ASM SM ASM ML ) using the following relationships for a
spherical cap: ASL 2r 2 1 cos , ASM r 2 sin 2 , and VS r 3 2 cos 1 cos / 3 .
2
10. (a) Show that surface melting is to be expected below Tm in gold (1336 K) given SL = 0.132,
LV = 1.128, SV = 1.400 J/m2.
(b) Given that the latent heat of fusion of gold is 1.2109 J/m3 estimate whether sensible liquid
layer thickness are feasible at measurably lower temperature than Tm.
14. Show
that
Equation
4.35
{
x
1 k
X L X 0 1
exp
k
D / v
}
satisfies
4.34
[ DC L' v C L C S ].
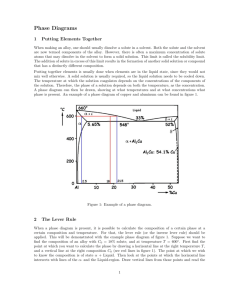
15. The Al-Cu phase diagram is similar to that shown in Fig. 4.19 with Tm(Al) = 660C, TE =
548C, Xmax = 5.65 wt%, and XE = 33 wt% Cu. The diffusion coefficient for the liquid DL =
310-9 m2/s. If an Al-0.5 wt% Cu alloy is solidifies with no convection and a planar
solid/liquid interface at 5 m/s:
(a) What is the interface temperature in the steady state?
(b) What is the thickness of the diffusion layer?
(c) What temperature gradient will be required to maintain a planar interface?
(d) Answer (a), (b) and (c) for an Al-2 wt% Cu alloy solidified under the same conditions.
16. (a) Using Equation 4.33 [ X L X 0 f Lk 1 ] and the data in problem 4.15 plot the variation of
copper concentration along a unidirectionally solidified bar of an Al-2 wt% Cu alloy assuming
no diffusion in the solid and perfect mixing in the liquid.
(b) What fraction of the bar will solidify to a eutectic structure?
(c) How much eutectic would form in an Al-0.5 wt% Cu alloy solidified under the same
conditions?
19. Show that the condition r 2r * gives (i) the maximum eutectic growth rate for a given
undercooling, and (ii) a minimum undercooling for a given growth rate (Equation 4.43:
v k 2 DT0
1 *
1 ).
20. Calculate the depression of the eutectic temperature for a lamellar eutectic with r = 0.2 m and
r = 1.0 m, if = 400 mJ/m2, H/Vm = 800106 mJ/m3, TE = 1000 K.
21. If it is assumed that the choice of a rod or lamellar eutectic is governed by the minimization of
the total / interfacial energy it can be shown that for a given there is a critical volume
fraction of the phase (fc) below which should be rod like, and above which it should be
lamellar. Assuming the rods are hexagonally arranged and that is isotropic, calculate the
value of fc.