Carbohydrates
advertisement

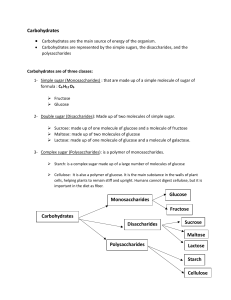
Carbohydrates What are Carbohydrates? Put simply…hydrocarbons. Empirical Formula CnH2nOn Divided into three groups Monosaccharides Disaccharides Polysaccharides Monosaccharides Monomers…single Let’s How sugar molecules. build glucose and fructose. are the molecules different? What functional groups are present? Monomers in Use Ribose and 2-deoxyribose are pentoses Used in DNA and RNA Glucose, hexoses Galactose, and Fructose are Used in plants and animals for energy Build into larger biological molecules like insulin. Disaccharides Two monosaccharides joined by a glycosidic bond. (ether) A water molecule is generated in the process of bond formation. (condensation polymerization) Disaccharides in Use Sucrose Lactose Table Sugar Let’s build it! Join the glucose and fructose from earlier. Milk sugar: glucose and galactose Lactose intolerant people lack the enzyme needed to break lactose’s glycosidic bond. Invert Sugar Bees use enzymes to break sucrose’s glycosidic bond to create invert sugar, which is the sweet flavor in honey. Polysaccharides Strings of more than 20 monosaccharides. Starch Major source of food calories consumed by people Amylose (in rice!)= linear polysaccharide Amylopectin = branched polysaccharide Glycogen = highly branched, stored in muscles and liver until converted to fat for long term storage. Alpha and Beta Linkages Starch and Cellulose are made of glucose. 2 isomer forms for linkagesalpha and beta. Starch has alpha linkages. (cis) Cellulose has beta linkages. (trans) Food uses of Carbohydrates Reducing sugars react with amino acids in the Maillard Reaction (causes browning) Polysaccharides bind water to thicken liquids into gels. Used to stabilize suspensions, emulsions. Pectin Pectin is a polysaccharide made of galacturonic acid (galactose) molecules with beta linkages. Found in green apples, lime peel, lemon peel When heated with sugar at low pH, branched polymerization occurs to create a thick gels.