ANTI-TUBERCULOSIS DRUGS
advertisement

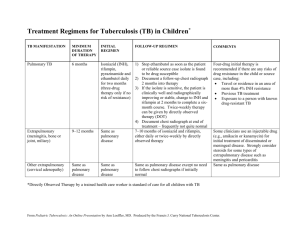
ANTI-TUBERCULOSIS DRUGS Tuberculosis (TB) is a disease that has affected mankind for centuries and dates as far back as ancient Egyptian times. is caused by a slow growing bacterium called M. tuberculosis. The disease is spread by coughing, talking, spitting or sneezing which spreads the mycobacteria through the air in tiny droplets of water which are inhaled in to the lungs. ANTI-TUBERCULOSIS DRUGS The primary location of the disease is in the lungs, called pulmonary TB, where bacterial growth destroys tissue making it very hard for the patient to breathe resulting in death. symptoms can also include meningitis, legions on the skin and degradation of the heart, bones and intestines. The difficulty in managing tuberculosis is the prolonged treatment duration, the emergence of drug resistance and co-infection with HIV/AIDS. ANTI-TUBERCULOSIS DRUGS Tuberculosis control requires new drugs that act at novel drug targets to help combat resistant forms of Mycobacterium tuberculosis and reduce treatment duration. Drug resistant strains of the mycobacterium are not susceptible to the current cocktail of drugs available. now infects approximately one third of the world’s population and causes 8 million new cases of TB each year resulting in around 2 million deaths worldwide. The resurgence has been caused due to three main reasons: The available chemotherapy is not very efficacious and has to be given as a precise combination over a period of months (poor compliance). 2. Drug resistant strains of the mycobacterium are not susceptible to the available drugs. 3. There is also a strong epidemiological coexistence between TB with HIV 1. Mycobacterium tuberculosis is the principal causative agent of TB in human. is a slow growing Gram-positive rod-shaped bacterium that has a thick, rigid, and hydrophobic cell wall which serves to protect the organism from the environment, making it highly impermeable to conventional antimicrobial agents. The current treatment of TB TB is treated in two phases. There is an initial phase and a continuation phase and depending upon the patients ability to comply with the drug regime: Phase-I: Here there is the concurrent use of at least three drugs to reduce the bacterial population as rapidly as possible in order to prevent resistance. 1. as a combination preparation or “triple therapy”. isoniazid (INH), rifampicin (RIF) and pyrazinamide (PZA). Streptomycin (SM) may be used in cases where resistance to INH has been established. The initial phase drugs are normally used for two months The current treatment of TB 2. Phase-II: After the initial phase, a further four months of chemotherapy is carried out using preferably a combination of RIF and INH. Potential targets in M. tuberculosis Targeted pathways should be unique to the Mycobacterium. Many agents have been introduced to target certain metabolic sites: 1. Inhibit cell wall biosynthesis. 2. Affect protein biosynthesis. 3. Affect DNA replication and transcription. 4. Inhibit fatty acid synthesis (FAS). Mycobacterial cell wall a very complicated cell wall which is sometimes described as “waxy” because of its complex fatty acid barrier which makes getting a drug molecule into the cytoplasm extremely difficult. Consists mainly from special type of fatty acids called mycolic acids which make mycobacterium exceptionally different from other gram +ve bacteria regarding the cell wall permeability. Mycobacterial cell wall Mycolic acids Mycolic acids OH O OH are β-hydroxy C54-63 fatty acids with a long α-alkyl side chain of C22-24 in length. play important roles in the mycobacterium, including resistance to chemical injury; resistance to dehydration, low permeability to polar molecules and allow the bacterium to grow readily inside macrophages α-mycolic acids are the predominant form (70%) Synthesis of α-mycolic acid Targeting mycolic acid biosynthesis Isoniazid (INH) – 1952 a purely synthetic agent. Has bacteriostatic action at lower dose and bactericidal at higher concentration. Inhibits fatty acid biosynthesis by interfering with InhA enzyme (essential one). It is a prodrug and has to be activated before killing the bacteria. Activation of INH isonicotinic acyl-NADH complex will tightly bound to the active site of InhA thus preventing access of the natural enoyl-AcpM substrate INH pharmacokinetic profile INH is well absorbed orally or intramuscularly and distributes well throughout the body (Why?). (LogP= -0.64). its metabolism occurs initially by liver Nacetyltransferase. This means that patients who are poor acetylators can experience toxicity problems as acetylation of INH is first required before the hydrolysis can occur and the drug cleared by the kidneys INH resistance Two major resistant mechanism have been reported: Resistance to INH generally occurs when the drug is administered alone for 3 months and is caused mainly due to the absence of the gene encoding the catalaseperoxidase katG which prevents activation of the drug. Mutations in InhA have also been identified and considered as a reason for resistance to the drug. Pyrazinamide (PZA) – 1952 drug is a synthetic analogue of nicotinamide. is bactericidal against growing bacteria. The exact mechanism of action of this unclear, but there are two proposed ones: Affect memebrane transport. Inhibit FAS-I system: This interferes with the bacterium’s ability to synthesize new fatty acids, required for growth and replication. it is known that PZA is a prodrug which requires activation to pyrazinoic acid (POA) by the Pyrazinamidase enzyme: Pyrazinamide pharmacokinetic profile PZA is well absorbed orally and distributes well throughout the body (Why?) reaching concentration levels above that needed to kill the tubercle bacilli. LogP = -0.42 It is metabolized by the liver to give mainly Pyrazinoic acid which is then excreted by the kidneys. It is also known that resistance to PZA rapidly evolves if the drug is used alone and is associated with mutation in the pncA gene which encodes the Pyrazinamidase/ nicotinamidase enzyme. Pyrazinamide More selective on M.tuberculosis than other mycobacterium species as well as other bacterial strains such as E. coli (Ying Zhang, 2008). This is thought to be due to effective efflux pump in those bacterial cells compared to M.tuberculosis which will pump PZA as pyrazinoic acid out of the cell. D-Cycloserine (CS) – 1952 possesses a broad range of anti-mycobacterial activity. It works by mimicking D-alanine which is the natural substrate for the enzyme D-alanine racemase (Alr) and D-alanine: D-alanine ligase (Ddl) thus preventing the synthesis of the mycolyl peptidoglycan which lead to cell death. D-Cycloserine pharmacokinetic and resistant profile CS is readily absorbed orally and widely distributed amongst tissues before being excreted by the kidneys with little of the drug being metabolized. Alr serves to convert L- aniline (bacteria utilize L- aniline from the host environment) to D-alanine and mutation in the active site of this enzyme have been proposed to be responsible for the resistance of CS. Not active against MDR-TB. Future TB drugs Recent research regarding anti-tuberculosis agents is interested in: 1. shortening treatment time. 2. combating MDR-TB. 3. and finding effective agents against persisting bacterial infections. Most of the work in this field focused in finding agents selective on inhibiting FAS-II system (specifically targeting new and essential enzymes), especially preventing mycolic acid biosynthesis. 2 1 3 Possible new targets for anti-TB agents 4 Case study for the development of new anti-TB agents. • TLM; a natural product form Nocardia, inhibits KasA and KasB (FAS-II). • Inactive on the whole bacteria (why?). • difficult to separate and synthesize. Molecular Modelling and Computer-Aided Drug Design Is the use of the three dimensional structure of macromolecular targets to design novel inhibitors. Then using computational docking programs to dock hypothetical structures into the binding site. the compounds then will be rank-ordered with respect to their goodness of fit. Compounds will be synthesized and tested on both the isolated enzyme and the whole bacterial cell. b OH c d OH OH Cl O O O O Cl Cl NH2 S O Cerulenin Triclosan Thiolactomycin N S NH2 S Ethionamide OH O B S N O N thienodiazoborine S O a Thiolactomycin O O Cerulenin Heath et al. 2001 O NH2 TLM-Enzyme 2-aminobenzimidazole-ecFabB 4-aminoimidazole-ecFabB Findings form modeling results TLM bound in the same pattern as reported in literature. Increasing the hydrophobic character of the compound improved the binding. The carbonyl group is essential for H-bonding. The in-vitro results showed promising activity in submicromolar range against M.tuberculosis.