Empirical Formula Lab
advertisement

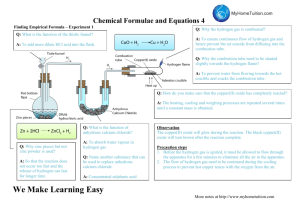
Name: ______________________________ Empirical Formula Determination of Magnesium Oxide and Copper Sulfate Purpose: The purpose of this lab is to experimentally determine the empirical formula of different chemical compounds using experimentally determined masses. ****NOTE****: Please make sure you download a copy of this lab before editing. Part 1: Combustion of Magnesium Pre-Lab Questions: 1. Write the balanced equation for the reaction between solid magnesium and oxygen gas to produce magnesium oxide. 2. What type of reaction is occurring? 3. Which reactant will be used up first? 4. Which reactant do you have more than enough of? WARNINGS: The crucible used in this lab will become VERY HOT and could cause severe burns if handled improperly Wear safety goggles at all times Do not look directly at the burning magnesium. Do not inhale the smoke/gas product when the magnesium is burning. Procedure: 1. Set up the experiment as directed by your teacher. Clean a crucible by wiping it dry with a paper towel. DO NOT use water to clean the crucible. Measure and record the mass of the crucible to the nearest 0.01 grams. 2. Measure out a small scoop of magnesium and place in the crucible. Measure and record the combined mass of the crucible and magnesium. Take a picture of the magnesium before heating. 3. Over a high flame with the crucible only partially covered, combust the magnesium thoroughly. You should be able to see the magnesium ignite and burn white to orange. Continue to heat the crucible for at least ten minutes or until the combustion process is complete. The magnesium should be converted to magnesium oxide, a white/light gray powder. 4. Turn the burner off. Allow the crucible to cool. While the crucible is cooling proceed to Part 2. Once completed with Part 2, return to Step 5 of Part 1. 5. Measure and record the combined mass of the crucible and magnesium oxide. Take a picture of the powder. 6. Rinse the powder thoroughly down the sink and clean out your crucible, making sure it’s dry. Clean up your lab station before proceeding with calculations. Data: Prepare and complete a data table for you measurements in the space below or attach an additional sheet of paper Calculations/Analysis Questions: 1. What signs do you have that a chemical reaction occurred? 2. Determine the mass of magnesium ribbon used. 3. Determine the number of moles of magnesium ribbon used. 4. Determine the mass of magnesium oxide formed. 5. Why are we able to complete the calculation in step #4? 6. Determine the mass of oxygen that combined with magnesium. 7. Calculate the number of moles of oxygen used. 8. Calculate the ratio of magnesium used to the amount of oxygen used. Express the ratio in simplest whole number form. 9. Based on the experimental data (and your answer to #7), what is the empirical formula for magnesium oxide? 10. Compare the experimentally determined formula to the known formula for magnesium oxide. 11. What are sources of error in this experiment? 12. At the high temperatures at which this reaction takes place, a green-yellow product may form. Propose a hypothesis about the chemical nature of this product. (HINT: Air is a mixture of different gases.) Researching online, determine what potential substance the Magnesium combined with instead of oxygen and the product that it formed (the green-yellow product). Write the balanced equation for this reaction. Part 2: Determination of Copper Sulfate Hydrate Formula Background Information: Recall that a HYDRATE is a compound that contains water as part of its structure and chemical formula. The water can be separated simply by heating up the substance which often results in a color change. The formula of the hydrate often indicates how many water molecules exist for every molecule of the actual compound. For example, Coblat(II) Chloride, often exists as a hexahydrate, meaning there are 6 molecules of water for every one molecule of Cobalt Chloride. The formula for this is written as 𝐶𝑜𝐶𝑙2 ∙ 6𝐻2 𝑂 Last time you were given a sample of Copper(II) Sulfate Hydrate, however, we do NOT know what type of hydrate it is, ie. we don’t know how many water molecules are in the formula. Unknown Copper Sulfate Hydrate = ____ 𝐶𝑢𝑆𝑂4 ∙ ___𝐻2 𝑂 ? Using the data you gathered from last class, answer the questions below to figure out the formula of the hydrate. 1) Fill in the chart of the data table below. Include units. Mass of the Copper(II) Sulfate Dehyrdated (without Water) Mass of the Water driven off 2). Using the molar mass of Copper(II) Sulfate and Water, determine the moles of each substance. 3) Divide each amount of moles by the smallest amount of moles. 4) The previous calculation will tell you many CuSO4 molecules and how many H2O molecules there are in the formula. Using those numbers, complete the formula below. Empirical Formula = ____ 𝐶𝑢𝑆𝑂4 ∙ ___𝐻2 𝑂