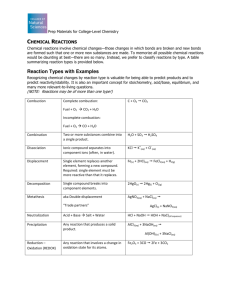
Combustion or Oxidation
advertisement

Combustion or Oxidation Names: 1. Mass of Mg __________g. 2. Mass of crucible and cover __________g. 3. Mass of crucible, cover and product __________g. 4. Mass of product __________g. 5. Mass of O2 __________g. 6. Does the product weigh more or less than before you burned the magnesium? Explain what happened. 7. What kind of a reaction is this? 8. Write a balanced chemical equation for this reaction 9. Figure out the mole amounts of the following reactants and products. Mg __________ O __________ MgO __________ 10. Diagram the Lewis Dot representation for this reaction. 11. Where does all the energy (brilliant light and heat) come from? 12. Compare/contrast this combustion reaction with the combustion of coal (pure carbon)? 13. If there was no oxygen present would the magnesium still burn? Explain. As part of your conclusion in your lab notebook diagram and explain the concept of oxidation/reduction with regards to this experiment being sure to identify what is being oxidized and what is being reduced.