Exemplar Conclusion and Evaluation Experiment Finding the
advertisement

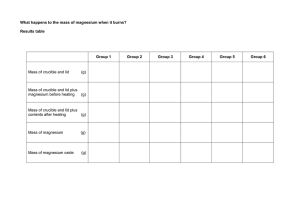
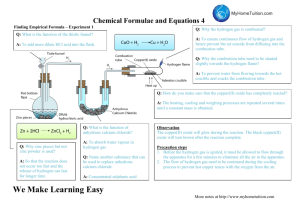
Exemplar Conclusion and Evaluation Experiment Finding the Empirical Formula of Magnesium Oxide IB Chemistry Note to Student: Anything below in bold-faced print is a note to you and not part of the actual laboratory write-up Background: This is related to the earlier data processing exemplar. The experiment involved measuring the mass of a Mg ribbon, then reacting it with oxygen over a Bunsen burner inside a crucible to make the product, magnesium oxide. The student would periodically have to peak inside the crucible to see if the reaction had quit. When the student lifted the lid, some of the magnesium oxide escaped. The required numbers are: Mg/O ratio from experiment = 1.32 +/- 24.5% Percentage error = 32% Conclusion: Based on the data processing above and the Mg/O ratio of 1.32, I would have to conclude from my experiment that the formula of magnesium oxide is Mg4O3. My method for determining this is as follows: Valid conclusion If the Mg/O ratio is 1.32, that means that the empirical formula would be Mg1.32O1. Obviously you cannot have a non-integral number in a chemical formula and 1.32 is too far to round down to 1.00, but multiplying it by 3 gives 3.96, which is almost 4.00. If you multiply Mg by 3, you must do the same to O, so the formula must be Mg4O3. Explanation of conclusion However, I know from the literature value of Mg/O = 1.00, that this must not be right. The literature value makes perfect sense because the reaction between magnesium and oxygen creates magnesium oxide, an ionic compound. Since the magnesium cation has a +2 charge and oxygen anion has a -2 charge, the real formula would have to be MgO, resulting in an Mg/O ratio of 1. Therefore, something is wrong with my experiment. Comparison to literature value and extension of explanation Evaluation: While the time required for the investigation and materials provided were sufficient, something seems to be wrong with the procedure. The percentage error of 32% is larger than the experimental uncertainty of 24.5%, so there must be significant systematic error. Compare error and uncertainty. Furthermore, because the percent error is positive, that means that there is either too much Mg or too little O. That is the only way that the ratio of Mg/O could be larger than 1.00. Something occurred to either inflate the amount of Mg represented, to lose O or possibly both. Use the value of the percent error and your method of analysis to point the way to possible systematic errors. The most likely cause of this error was the fact that the procedure had us lift the lid of the crucible and look inside to check and see if the reaction was finished every two minutes. When I lifted the lid of the crucible, some white smoke escaped. This was our product, magnesium oxide. Since we had already recorded the mass of the magnesium before the reaction started, any loss of product would make it appear as though oxygen was lost. It would, in effect, make the oxygen part of the Mg/O ratio too small and thereby produce a number larger than 1.00. That is exactly what we saw. Identify the specific error(s)/weakness(s) and explain how it would produce the results you obtained. While it is necessary to see if the reaction is finished, and therefore some loss of product is unavoidable, I do not think that the procedure made it clear just what impact this could have on the results. I also think that checking every two minutes was excessive. Therefore, in the future I think that this error could be minimized by placing an explicit warning in the procedure and by increasing the checking time to every five minutes instead of two. Realistic modification to the experiment that explicitly addresses the error(s)/weakness(s) you identified. There are other problems that could have caused this specific error, such as accidentally losing some of the product when moving the crucible to the balance to weight it at the end. Losing product this way would also cause the oxygen part of the Mg/O ratio to appear to be too small in exactly the same way as explained above. The only solution to this problem would be to ensure that the lid is on the crucible and to carry it as carefully as possible. While I think that we did this, we might have inadvertently made this mistake. It is good to have more than one possible error/weakness when you can!!