Solutions for Lab 8
advertisement

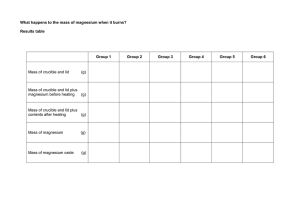
Solutions for ChemLab 8: Making Ionic Compound Pre-Lab: 1. Variable = mass of magnesium Constants = excess of oxygen and nitrogen 2. Mg = [Ne]3s2 a. lose 2 electrons b. [Ne]+2 or 1s22s22p6 +2 c. neon 3. O = [He]2s22p4 N = [He]2s22p3 a. O will gain 2 electrons; N will gain 3 b. oxygen = [He]2s22p6 -2 or [Ne]-2 nitrogen = [He]2s22p6 -3 or [Ne]-3 c. both will have neon’s configuration 5. The masses of the crucible empty, before and after are direct measurements, but the masses of the Mg and Mg product are calculated. 6. The masses of the Mg and Mg product is done by subtracting the mass of the empty crucible from the before and after mass of the crucible. Analyze & Conclude: 1. The mass of the products should have been greater. 2. Heat and light, so the resulting product is more stable. 3. There was an increase in mass and other signs of a chemical reaction. 4. MgO = magnesium oxide Mg3N2 = magnesium nitride 5. MgO because the product appears white 6. yes, so the products must be ionic 7. some of the product blew out of the crucible or the reaction was incomplete