Phase diagrams
advertisement

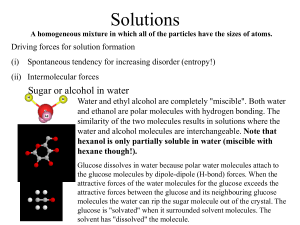
Vapour Pressure and Heat What happens to a solid substance when it is heated? The compound can simply get hotter or a phase change can occur. The transition from the solid phase to the liquid phase is an example of a phase change, which is often called melting. Boiling or vapourisation is an example of a phase change from the liquid to the gas phase. Phase changes can be expressed as enthalpy changes at constant temperatures (Claussius-Clapeyron equation). vapH ln P o P R 1 1 o T T Phase diagrams Show regions of P and T at which various phases are thermodynamically stable Triple point: three phases in equilibrium Vp curve for solid Vp curve for liquid Melting point line Critical point Supercritical fluids Heating liquid in a closed vessel does not produce boiling. The vp (density) of the vapour rises with increasing T, as the liquid density decreases, until both are equal and a single phase exists (neither liq nor vap.). Typical phase diagrams Water Carbon dioxide Triple pt: 6.11mbar, 273.16K Triple pt: 5.11bar, 218.8K Critical pt: 215bar, 647.3K Critical pt: 72bar, 304.2K Solutions A homogeneous mixture in which all of the particles have the sizes of atoms. Driving forces for solution formation (i) Spontaneous tendency for increasing disorder (entropy!) (ii) Intermolecular forces Sugar or alcohol in water Glucose has -O-H groups along the carbon skeleton. These -O-H are polar centers. Glucose dissolves in water because polar water molecules attach to the glucose molecules by dipole-dipole (H-bond) forces. When the attractive forces of the water molecules for the glucose exceeds the attractive forces between the glucose and its neighbouring glucose molecules the water can rip the sugar molecule out of the crystal. The glucose is "solvated" when it surrounded solvent molecules. The solvent has "dissolved" the molecule. Water and ethyl alcohol are completely "miscible". Both water and ethanol are polar molecules with hydrogen bonding. The similarity of the two molecules results in solutions where the water and alcohol molecules are interchangeable. Heats of Solution The enthalpy change between system and surroundings when 1 mole of a solute dissolves in solvent at constant pressure Vapourised particles and solvent solvation energy -lattice energy solnH solid solution solvent Heat of solution is zero for an ideal solution Ideal dilute solutions PB = xBKB Henry’s Law Colligative Properties of Solutions Physical properties that depend only upon the populations of particles in a mixture Effect of solutes on the vapour pressure of solutions Psoln = xsolventP*solvent Raoult’s Law Molecular interpretation of Raoult’s Law Volatile solutes and Raoult’s Law Each component contributes its own partial pressure to the solution vapour pressure (Dalton’s Law) Real mixtures Deviations because of intermolecular attractions Boiling point elevation Freezing point depression Entropy effect: when a solute is added to a pure liquid, the entropy is increased relative to the vapour phase. Therefore there is a weaker tendency to form a vapour (boiling point elevation). A similar molecular interpretation explains freezing point depression. T f K f m Tb K b m Osmotic pressure Osmotic membrane: semi-permeable membrane that allows passage of only solvent molecules Dialysis membrane: membrane that allows passage of solvent and small solutes. Van’t Hoff equation MRT Colligative properties of solutions of electrolytes 1.00 m NaCl: F.P= -3.37C (not –1.86C as expected)! Colligative properties depend on the concentration of particles Remember: NaCl Na+ + ClWe have 2.00m of particles and should get F.P: -(2x1.86C) = -3.72C Effect of interionic attractions account for discrepancy between actual and calculated F.P. for ionic species. Van’t Hoff Factor compares degrees of dissociation of electrolytes Tmeasured i Tca lcu la ted