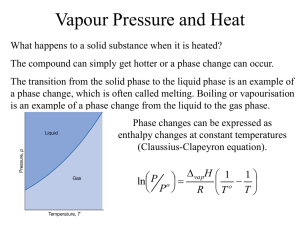
Phase diagrams

Solutions
A homogeneous mixture in which all of the particles have the sizes of atoms.
Driving forces for solution formation
(i) Spontaneous tendency for increasing disorder (entropy!)
(ii) Intermolecular forces
Sugar or alcohol in water
Water and ethyl alcohol are completely "miscible". Both water and ethanol are polar molecules with hydrogen bonding. The similarity of the two molecules results in solutions where the water and alcohol molecules are interchangeable. Note that hexanol is only partially soluble in water (miscible with hexane though!).
Glucose dissolves in water because polar water molecules attach to the glucose molecules by dipole-dipole (H-bond) forces. When the attractive forces of the water molecules for the glucose exceeds the attractive forces between the glucose and its neighbouring glucose molecules the water can rip the sugar molecule out of the crystal. The glucose is "solvated" when it surrounded solvent molecules. The solvent has "dissolved" the molecule.
Solution formation
Alcohol (ethanol) in water
Engine coolants
• We can now explain why car radiator coolants dissolve in water. The coolants typically contain either ethylene glycol or propylene glycol, which, like ethanol and water, contain hydrogen-bonding O-H bonds.
Solublility & Miscibility
(like dissolves like)
How do stain removers work?
• Like dissolves like dry cleaning, surfactants and oxidizing agents
(and enzymes!)
Energy Changes and Solution Formation
Heat of solution: The enthalpy change between system and surroundings when 1 mole of a solute dissolves in solvent at constant pressure
In general, solutions form when
H soln is negative.
-When
H soln is too positive, a solution will not form.
-Heat of solution is zero for an ideal solution
Examples:
MgSO
NH
4
4
NO
3 added to water has
H soln added to water has
H soln
= -91.2 kJ/mol.
= + 26.4 kJ/mol.
MgSO
4 is used in hot packs and NH
4
NO
3 is used in cold packs.
Ideal dilute solutions-effect of pressure on solubility
P
B
= x
B
K
B
Pressure and Solubility
• “The Bends” - deep sea divers
– N
2 in compressed air has low solubility in blood. At great depths, partial pressure of N
2 increases and solubility increases. After returning to surface, solubility decreases substantially and N
2 come out of blood causing small bubbles in the capillaries which may rupture the blood vessels - fatal - risk reduced if He is used since it has lower Henry’s constant.
• Soft drinks and champagne
– produced by dissolving CO
2 in a liquid under pressure
– opening bottle reduces the partial pressure of CO
2 solubility reduced, CO
2 above solution, leaves solution (effervescence)
Colligative Properties of Solutions
Physical properties that depend only upon the populations of particles in a mixture
Effect of solutes on the vapour pressure of solutions
P soln
= x solvent
P * solvent
Raoult’s Law
Molecular interpretation of Raoult’s Law
Volatile solutes and Raoult’s Law
Each component contributes its own partial pressure to the solution vapour pressure
(Dalton’s Law)
Real mixtures
Deviations because of intermolecular attractions
Application:
Distillation
Distillation
Boiling point elevation
Freezing point depression
Entropy effect (see later): when a solute is added to a pure liquid, the entropy
(disorder) is increased relative to the vapour phase. Therefore there is a weaker tendency to form a vapour (boiling point elevation). A similar molecular interpretation explains freezing point depression. The effect shifts the s-l and l-g vp curve of a phase diagram down.
T f
T b
K f m
K b m
Applications:
Cooking
De-icing and anti-freeze
Molar mass determination
Osmotic pressure
Osmotic membrane: semi-permeable membrane that allows passage of only solvent molecules
Dialysis membrane: membrane that allows passage of solvent and small solutes.
Van’t Hoff equation
MRT
Applications:
Reverse osmosis
Crenation and hemolysis of red blood cells
Molar Mass determination
Colligative properties of solutions of electrolytes
1.00 m NaCl : F.P= -3.37C (not –1.86C as expected)!
Colligative properties depend on the concentration of particles
Remember: NaCl Na + + Cl -
We have 2.00m
of particles and should get F.P: -(2x1.86C) = -3.72C
Effect of interionic attractions account for discrepancy between actual and calculated
F.P. for ionic species.
Van’t Hoff Factor compares degrees of dissociation of electrolytes i
T mea su red
T ca lcu la ted