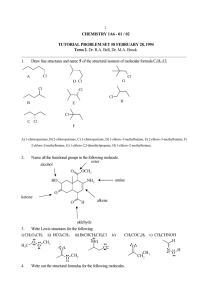
ISOMERISM
advertisement

ISOMERISM There are two main types of isomerism Structural i.e. molecular positional functional group Stereoisomerism i.e. Geometric and Optical Molecular • This is something you are already aware of from previous work. • i.e. • CH3 • CH3CH2CH2CH3 CH3CHCH3 • Butane 2-methylpropane Positional • You are also aware of most of these from alcohols and halogenoalkanes • e.g. • Cl • CH3CH2CH2OH CH3CHCH3 • Propan-1-ol 2-chloropropane • However we need to consider aromatics Positional (aromatics) • X X • X • • X • • 1,2- (ortho) 1,3- (meta) X X 1,4- (para) Functional group • There are three main pairs of homologues involved here, namely:• 1. Alcohols and ethers (alkoxyalkanes) • 2. Alkanals and alkanones • 3. Alkanoic acids and esters Functional group • • • • • • Alcohols and alkoxyalkanes e.g. For the general molecular formula C2H6O it is possible to have:CH3CH2OH and CH3OCH3 Ethanol Methoxymethane Problem • Write out all possible isomers of C4H10O together with their systematic names. • CH3CH2CH2CH2OH • Butan-1-ol • CH3 • CH3CHCH2OH • 2-methylpropan-1-ol C4H10O • • • • • • • CH3CH2CHCH3 OH Butan-2-ol CH3 CH3CCH3 OH 2-methylpropan-2-ol C4H10O (continued) • CH3CH2OCH2CH3 • Ethoxyethane (diethyl ether) • CH3OCH2CH2CH3 • Methoxypropane (methyl propyl ether) Alkanals and alkanones • For the general molecular formula C3H6O it is possible to have:• CH3CH2CHO • Propanal and CH3COCH3 Propanone Problem • Write out all possible isomers of C4H8O together with their systematic names • CH3CH2CH2CHO CH3CHCHO • CH3 • Butanal 2-methylpropanal • CH3CH2COCH3 • Butanone Alkanoic acids and esters • For the general molecular formula C2H4O2 it is possible to have • CH3COOH • Ethanoic acid and HCOOCH3 Methyl methanoate Problem • Write out all possible isomers of C4H8O2 together with their systematic names • CH3CH2CH2COOH CH3CHCOOH • CH3 • Butanoic acid 2-methylpropanoic acid • C4H8O2 (continued) • • • • • • • CH3COOCH2CH3 Ethyl ethanaoate CH3CH2COOCH3 Methyl propanoate HCOOCH2CH2CH3 Propyl methanoate There is one other ester which occurs:- (continued) • • • • HCOOCHCH3 CH3 2-methylethyl methanoate