1 CHEMISTRY 1A6 - 01 / 02 TUTORIAL PROBLEM SET #8
advertisement
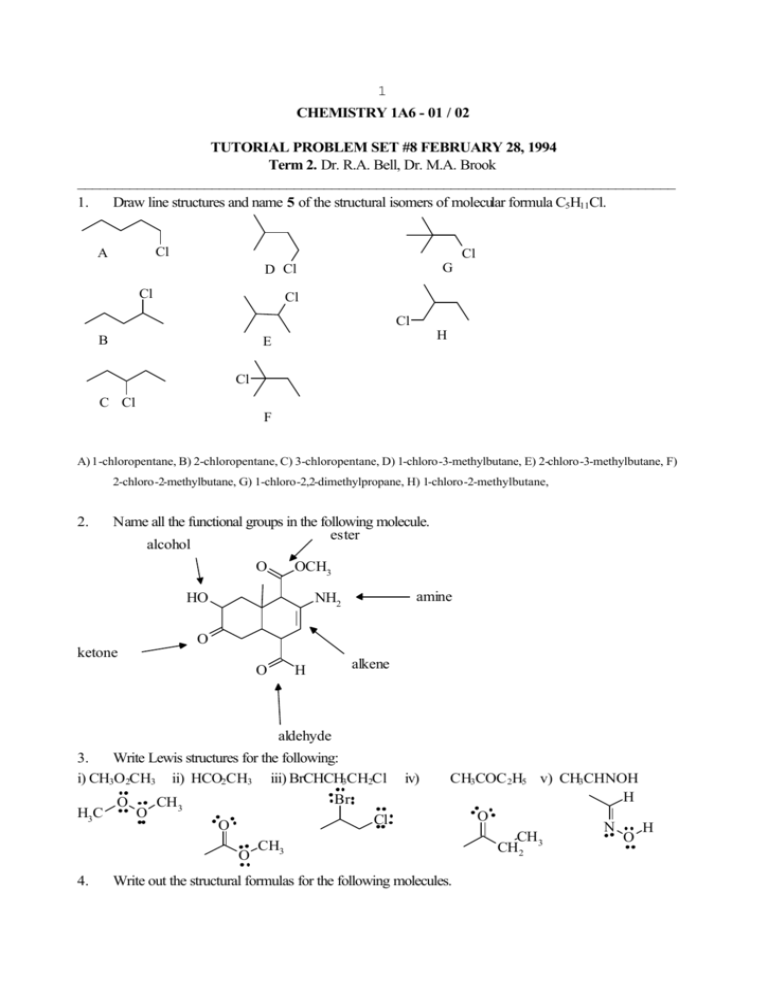
1 CHEMISTRY 1A6 - 01 / 02 TUTORIAL PROBLEM SET #8 FEBRUARY 28, 1994 Term 2. Dr. R.A. Bell, Dr. M.A. Brook ________________________________________________________________________________ 1. Draw line structures and name 5 of the structural isomers of molecular formula C5H11Cl. Cl A Cl G D Cl Cl Cl Cl B H E Cl C Cl F A) 1-chloropentane, B) 2-chloropentane, C) 3-chloropentane, D) 1-chloro-3-methylbutane, E) 2-chloro-3-methylbutane, F) 2-chloro-2-methylbutane, G) 1-chloro-2,2-dimethylpropane, H) 1-chloro-2-methylbutane, 2. Name all the functional groups in the following molecule. ester alcohol O OCH3 HO amine NH2 O ketone O H alkene aldehyde 3. Write Lewis structures for the following: i) CH3O2CH3 ii) HCO2CH3 iii) BrCHCH3CH2Cl Br O CH3 H3C O Cl O O 4. CH3 iv) CH3COC 2H5 v) CH3CHNOH H O N H CH3 O CH2 Write out the structural formulas for the following molecules. 2 i) 2-amino-1-hydroxybutane methylhexane ii) cis-1-chloro-3-methylcyclopentane iii) 2-bromo-3-chloro-3Br Cl OH CH3 NH2 5. Cl Determine the E or Z geometric configurations for the following alkenes and name them. H H3CH2C OCH 3 i) E-cyclodecane 6. CH3 H3C Br iii) ii) E-2-bromobut-2-ene Z-2-methoxy -3-methylpent-2-ene Draw Newman projections for 1-propanol looking along the C1-C2 bond. Label each conformer as anti or gauche. Which of these three would you expect to be the most stable. CH3 CH3 CH3 H H H OH H H H H OH H H H H H gauche anti gauche HO The anti is more stable. 7. Write out all the structural and geometric isomers (stereoisomers) of chloromethylcyclohexane. Use planar projections formulae and indicate the orientation (stereochemistry) of the Cl and Me groups by using a wedged line (out of the plane of the page) and dashed line (below the plane of the page). Label each of the isomers are cis or trans. Cl not relevent Cl Cl Cl trans cis trans Cl Cl Cl cis trans cis