PPT
advertisement

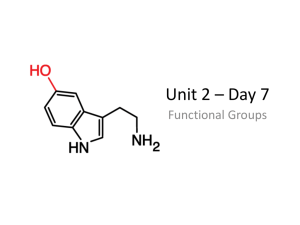
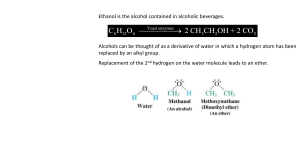
GSCI 163 Lecture 13 Derivatives of hydrocarbons Carbon can bond to one another and hydrogen, but they can also bond to other atoms and molecules. When they bond to other atoms and molecules besides hydrogen, they form derivatives molecules. How do we classify the enormous number of compounds derived from hydrocarbons? Functional group The hydrocarbon structure function as a framework for the attachment of other atoms. These atoms are called heteroatoms (hetero = different). The heteroatoms give character to an organic molecule. Combined they form a functional group that behave as a unit. Example: ethane ethanol ethylamine Alcohols Hydroxyl group Alcohols are soluble in water (why?) Structure Scientific name Common name Melting point (C) Boiling point(C) Methanol Methyl alcohol -97 65 Ethanol Ethyl alcohol -115 78 2-Propanol Isopropyl alcohol -126 97 Phenols Phenolic group It is acidic because it readily loses an H+ in solution. What happens with the electron left? Circles around the benzene ring First antiseptic for its anti-bacterial properties (Joseph Lister, 1867) Ethers Ether group Related to alcohols, but with the oxygen bonded to two carbons. In contrast with alcohols they are not very soluble in water. Why? The absence of the hydroxyl group. Most famous compound: diethyl ether The first anesthetic. Characteristics: volatile, low solubility in water Amine and alkaline Amine group Less soluble in water, lower boiling point as compared to alcohols. Alkaline because they readily accepts hydrogen ion Notable properties (low formula mass): offensive odor Putrescine Cadaverine Carbonyl group Carbon atom A example of a ketone is Acetone, the fingernail polish remover. double bonded to oxygen Many have a particular fragrance; flowers for example owe their pleasant odor to the presence of simple aldehydes. One example is vanillin that give the pleasant vanilla taste. Amide and carboxyl group DEET - repellent Acetic acid - vinegar Esters and their flavors and odors: Building blocks of life Amino acids is an organic compound that contains both an amino group and carboxyl group. There are over 20 known amino acids, 8 of which are essential to life. Proteins are a long chain of amino acids bound together. The linkage process of each block is performed by the nucleic acids, which are themselves the made of amino and carboxyl groups. Polymers Blocks of monomers chained together: Teflon, for example, looks like a chain of ethylanes where the hydrogen has been replaced by fluoride atoms: Next class • Final exam