Functional Groups
advertisement
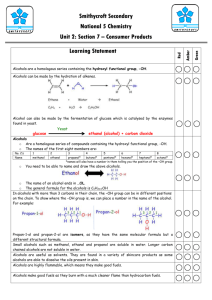
Unit 2 – Day 7 Functional Groups Functional Groups • Functional groups are groups of atoms that affect the way hydrocarbons behave. • They replace a hydrogen on the hydrocarbon. Methanol Methane Organic Families • If hydrocarbons have the same functional groups, they tend to behave the same way. • For this reason, organic families of chemicals are grouped based on functional groups. Organic Families Functional Group Family Name Examples -OH Hydroxyl Group Alcohols Rubbing Alcohol Grain Alcohol -COOH Carboxyl Group Carboxyllic Acids Vinegar -C=O Carbonyl Group Ketones (mid) Aldehydes (end) Formaldehyde Acetone -NH2 Amino Group Amines Antihistamines, Amino Acids Organic Families Functional Group Family Name -OEther Examples Ethers Diethyl ether Esters Flavours and Odours C=C Alkenes Ethylene CC Alkynes Acetylene Ester Alcohols • All alcohols have the hydroxyl group. • This is a polar covalent bond, which allows alcohols to be volatile liquids. • They are all toxic and flammable. Naming Alcohols • We name alcohols using the same prefix and suffix system used for hydrocarbons. • The prefix indicates the number of carbons. • All alcohols end in “–anol” • We use a number to indicate which carbon has the hydroxyl group. Examples: • CH3-CH2OH • CH2OH-CH2-CH2-CH3 • Draw 2-pentanol Carboxylic Acids • Carboxylic acids all contain the carboxyl group. • This group easily loses its hydrogen atom in water, which makes these molecules acidic. • They are corrosive like other acids, and also volatile like organic compounds. • One example is vinegar (acetic acid) Naming Carboxylic Acids • We name acids using the same system. • The prefix indicates the number of carbons. • The suffix for all carboxylic acids is “-anoic acid”. • The carboxyl group can only go on the end carbon, so no number is required. Examples: • CHOOH • CH3CH2COOH • Draw butanoic acid