Water molecule
advertisement

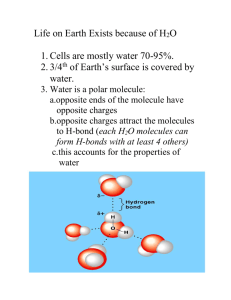
TUMS Azin Nowrouzi, PhD 1 Importance of water 1. The most abundant substance in living systems. – 2. Makes up 70% or more of the weight of most organisms. Living organisms depend on water for their existence. – Physical & chemical properties of water make it fit to support life: • • 3. 4. 5. 6. 7. High boiling point water remains liquid in most seasons. Ice less dense than water floats on liquid water and water freezes from top to bottom. So a good insulator: a frozen layer of ice serves as a blanket that protects creatures below. Ubiquitous solvent in cells. Excellent solvent of polar and ionic substances. Medium where most cell’s metabolic reactions take place. Ionization of water and its acid-base reactions important for the functions of proteins and nucleic acids. The Shapes of proteins and nucleic acids and structure of biological membranes are consequence of their interaction with water. 2 WATER Microscopic Structure of waster Water molecule: Polarity WeakInteractions • H-bonds • • • Crystals Bound water H-bonds between •Water molecules and other molecules •Between dissolved molecules Ionization of Water, • Weak Acids • Weak Base • pH Water as a solvent: Buffering against pH Changes • • • Biological buffers Water as a reactant Ions Polar substances Non-polar substances 3 “Water molecule” is polar • Water has a simple structure. • Oxygen has 6 electrons in the outer shell: 1s2, 2s2, 2p4. • Sp3 hybridization H—O—H bond angle is 104.5. • The net charge of water molecule is zero. But O—H bond is polar because O is more electronegative than H. Sharing of electrons between H and O is unequal. • The charge on O = -0.82 and on H = +0.41. This charge separation produces permanent dipoles. 4 Strength of H-bond • H-bond can form when a H atom that is bonded to O or N lies within 0.27-0.30 nm of another O or N atom that has unshared e-. 5 Water molecules form H bond • Attraction between positive H and negative O of another H2O produces dipole-dipole attraction called a hydrogen bond (H bond). • H bond is 10% covalent and 90% electrostatic. • The strength of H-bond is 4 kcal/mol, less than 5% as strong as a typical covalent bond. The strength of O—H bond is 110 kcal/mol. H-bond is a weak interaction. • Notice that the hydrogen bond (shown by the dashed line) is somewhat longer (117 pm) than the covalent O—H bond (99 pm). This means that it is considerably weaker. 6 Some biologically important H-bonds 7 Structure of water in ice • At any given time, most of the molecules in water are engaged in hydrogen bonding, but the lifetime of each hydrogen bond is just 1-20 picoseconds (1ps = 10-12s). • In liquid water, molecules are disorganized and in continuous motion, so that each molecule forms H-bonds with an average of only 3.4 other molecules. • In ice crystal each H2O is hydrogen bonded to 4 other H2O molecules. 8 Microscopic structure of water • Short-lived groups of water molecules interlinked by hydrogen bonds in liquid water are called flickering clusters. more dense water less dense water 9 Structured water 2(H2O)4 More dense water (H2O)8 Less dense water Suggested by Prof. Martin Chaplin of the London South Bank University 10 Structured Water Is Changing Models (H2O)8 (H2O)14 (H2O)17 (H2O)21 (H2O)196 (H2O)280 (H2O)more Bennett Daviss The Scientist 2004, 18(21):14. (H2O)280 11 • At any given time, most of the molecules in liquid water are engaged in hydrogen bonding, but the lifetime of each hydrogen bond is just 1 to 20 picoseconds (1 ps = 10-12 s). • Upon breakage of one hydrogen bond, another hydrogen bond forms, with the same partner or a new one, within 0.1 ps. • The apt phrase “flickering clusters” has been applied to the short-lived groups of water molecules interlinked by hydrogen bonds in liquid water. • The sum of all the hydrogen bonds between H2O molecules confers great internal cohesion on liquid water. 12 كريستالهاي منجمدشده آب The messages from water 13 سالم 14 (كاواچي ) رقص سنتي ژاپني “Goldberg Variations آهنگ باخ water can have highly organized local structures when it interacts with molecules capable of imposing these structures on the water. William Royer Jr. U. of Mass. Medical school Organized water molecules India, 2003 15 Stabilization by “bound water” molecules a b • Water binding in hemoglobin The crystal structure of hemoglobin, shown (a) with bound water molecules (red spheres) and (b) without the water molecules B-DNA with a spine of water molecules 16 ‘Bound water’ in biological systems • Intracellular water very close to any membrane or organelle (sometimes called vicinal water) • Organized very differently from bulk water • This structured water plays a significant role in governing the shape (and thus biological activity) of large folded biopolymers. 17 Water chain in cytochrome f 18 Proton hopping 19 Water molecules form H-bonds with polar solutes 20 Electrostatic interaction with charged solutes • When NaCl is mixed with water, a shell of water surrounds each Na+ and Cl- ion. 21 Ions change structure of liquid water • Ionic substances are soluble because the net attraction of the + and – ions for water is greater than the attraction of oppositely charged ions for each other. • Formation of the Hydration shell. 22 Structured water 2(H2O)4 More dense water (H2O)8 Less dense water 23 Types of ions • Structure-breaking ion 'chaotrope' (disorder-maker) (Na+) • structure-forming ion 'kosmotrope' (order-maker) (K+) • Kosmotropes shift the local equilibrium to the right. • Chaotropes shift it to the left. more dense (condensed) water less dense water Water preference Ion Surface charge density Intra-cellular Extra-cellular Ca2+ 2.11 0.1 mM 2.5 mM High density Na+ 1.00 10 mM 150 mM High density K+ 0.56 159 mM 4 mM Low density 24 Nonpolar gases are poorly soluble in water 25 Hydrocarbons in water • Hydrocarbons and nonpolar molecules are insoluble because water-water interactions are stronger than water-hydrocarbon interactions. So water molecules force nonpolar molecules together and surround them. • This phenomenon is called hydrophobic effect or hydrophobic interaction. 26 27 Water as a solvent Water is a polar solvent: • Compounds that dissolve easily in water are hydrophilic (Greek, “water-loving). • Nonpolar molecules that are not dissolved in water can be dissolved in nonpolar solvents (chloroform, and benzene). These nonpolar molecules are called hydrophobic (waterfearing). • Compounds that contain regions that are polar (or charged) and regions that are nonpolar are called amphipatic or amphiphilic. 28 Water undergoes ionization • Water is the biological solvent. • Pure water contains not only H2O, but also equal concentrations of hydrated protons, hydronium ions (H3O+), and hydroxide ions. 29 Ionization of water Keq = 1.8 x 10-16 [H2O]=55.5 M because (1000g/L)/(18.015 g/mol) (55.6)Keq = [H+][OH-] (55.6)(1.8 x 10-16) = [H+][OH-] = Kw Kw = 1.0 x 10-14 = [H+][OH-]= [H+]2 [H+] = [OH-] = = 1.0 x 10-7 M [H+] = [OH-] Neutral solution [H+] > [OH-] Acidic solution [H+] < [OH-] Basic (alkaline) solution 30 The pH of some aqueous fluids. 31 Dissociation of weak acids • An acid is a chemical species that can donate a proton (H+), and a base is a species that can accept (gain) a proton, according to the common Brnstead-Lowry definition. 32 Functions of [H+] • [H+] and therefore pH is important for many biochemical processes: – Transport of oxygen in blood – Enzymatic reactions – Generation of metabolic energy during respiration and photosynthesis – Metabolism and its regulation. – Measurement of pH of blood and urine are commonly used in medical diagnoses. 33 Types of acids 34 Titration curve 35 36 Buffers & Buffer Systems • Buffers are aqueous systems that tend to resist changes in pH when small amounts of acid (H+) or base (OH-) are added. • Buffers include weak acids that can donate hydrogen ions and weak bases that can absorb them. • For example, a mixture of equal concentrations of acetic acid and acetate ion found at the midpoint of the titration curve: acetic acid-acetate buffer pair Here the buffering power of the system is maximal 37 Example: Cpmparison of buffered and unbuffered solutions Add to each 1mmol of solid NaOH. • [OH-] = 1.0 mmol/100ml = 0.01M pOH = 2.00 pH = 12.0 • The change of pH is from 7.0 to 12.0 or 5 units. • 1.0 mmol of OH- reacts with 1.0 mmol of HA. mmol of HA decreases to 9.0. mmol of A- increases to 11.0. pH = 7.0 + log 11/9 pH = 7.09 • The change of pH is only from 7.00 to 38 7.09 or 0.09 units. How does a buffer system work? • Buffering results from two reversible reaction equilibria. • It occurs in a solution of nearly equal concentrations of a proton donor and its conjugate proton acceptor. • The decrease in concentration of one component of the system is balance exactly by an increase in the other. 39 The Henderson-Hasselbalch equation • Important for understanding buffer action and acid-base balance in the blood and tissues. • Calculation of pH, pKa and the molar ratio of proton donor and acceptor. • Shows why pH=pKa at the midpoint of titration. 40 Body buffer systems A. Blood buffers 1. plasma buffers 1. Bicarbonate buffer 2. Hemoglobin, HCO3-, and phosphate in erythrocytes. B. Tissue buffers A. Muscle B. Bone C. Intracellular buffer system A. Phosphate buffer system B. Protein buffer system D. Urine buffers A. Phosphate buffer system B. Ammonia buffer 41 Important buffers at physiologic [H+] 42 Bicarbonate/carbonic acid buffer system CO2 as an acid 1. The greatest source of H+. 2. End product of oxidation of glucose and triglyceride during aerobic metabolism. 3. Most important weak acid in body fluids. 43 Hemoglobin in RBCs • Hemoglobin protein can reversibly bind either H+ (to the protein) or O2 (to the Fe of the heme group), but that when one of these substances is bound, the other is released (as explained by the Bohr effect). • During exercise, hemoglobin helps to control the pH of the blood by binding some of the excess protons that are generated in the muscles. • At the same time, molecular oxygen is released for use by the muscles. 44 2. Phosphate/phosphoric acid buffer sytem • Phosphate concentration in the blood is so low that it is quantitatively unimportant. • Phosphates are important buffers intracellularly and in urine where their concentration is higher. • Phosphoric acid is triprotic acid that exists in four forms. Phosphoric acid has a pKa value for each of the three dissociations with three pK values of 2.1, 6.8, and 12.7: H3PO4 ↔ H2PO4- ↔ HPO4-2 ↔ PO4-3 • When a solution of phosphoric acid (H3PO4) is titrated completely, there will exist three buffering regions. • Physiologically, we only need to deal with the pH range between pH 4.5 (acidic urine) and 8.0 (alkaline urine). • For this reason, only two forms of phosphate (H2PO4- and HPO4-2) and one pK value (6.8). • H2PO4- is the acid form, and HPO4-2 is the salt form. 45 Acid-base disturbances • • • • The condition called respiratory acidosis occurs when blood pH falls as a result of decreased respiration. When respiration is restricted, the concentration of dissolved carbon dioxide in the blood increases, making the blood too acidic. Such a condition can be produced by asthma, pneumonia, emphysema, or inhaling smoke. Metabolic acidosis is the decrease in blood pH that results when excessive amounts of acidic substances are released into the blood. This can happen through prolonged physical exertion, by diabetes, or restricted food intake. The normal body response to this condition is increases breathing to reduce the amount of dissolved carbon dioxide in the blood. This is why we breathe more heavily after climbing several flights of stairs. Respiratory alkalosis results from excessive breathing that produces an increase in blood pH. Hyperventilation causes too much dissolved carbon dioxide to be removed from the blood, which decreases the carbonic acid concentration, which raises the blood pH. Often, the body of a hyperventilating person will react by fainting, which slows the breathing. Metabolic alkalosis is an increase in blood pH resulting from the release of alkaline materials into the blood. This can result from the ingestion of alkaline materials, and through overuse of diuretics. Again, the body usually responds to this condition by slowing breathing, possibly through fainting. 46 Water as a reactant (metabolic water) 47 • Cybotactic region: That part of a solution in the vicinity of a solute molecule in which the ordering of the solvent molecules is modified by the presence of the solute molecule. The term solvent "cosphere" of the solute has also been used. KOSOWER (1968); STEWART and MORROW (1927) 48