Water is a polar molecule
advertisement

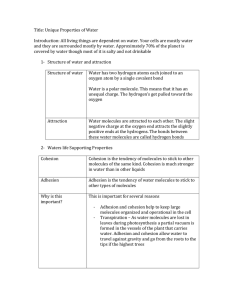
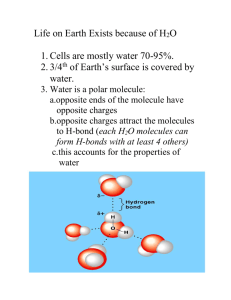
Water Importance of Water More than 70% of our total body weight is water Necessary for photosynthesis H becomes incorporated into organic compounds Oxygen released for us to breathe Solvent for most biochemical reactions Important reactant/product Water is a polar molecule Due to differences in electronegativities, water is slightly charged at its poles Oxygen takes on a slight – charge Hydrogens take on a slight + charge http://programs.northlandcollege.edu/biology/ Biology1111/animations/hydrogenbonds.html Hydrogen Bonding Water can form H-bonds with up to 4 neighboring water molecules It is water’s polarity that gives it many of its unique properties Less dense as a solid than a liquid Universal solvent Adhesion and cohesion Capillary action Surface tension High heat of vaporization High specific heat Water is the universal solvent Water can dissolve many hydrophilic substances Ionic compounds Other polar compounds Form “spheres of hydration” http://www.mhhe.com/physsci/chemistry/esse ntialchemistry/flash/molvie1.swf Some substances do not dissolve readily in water Hydrophobic – “water-fearing” Non-polar substances like lipids Cohesion Water molecules have a strong tendency to stick to one another Cohesion allows water to have a high degree of surface tension Any force is transmitted to the column of water as a whole Adhesion Ability of water to stick to other substances, esp. charged atoms or molecules Together, cohesion and adhesion allow for capillary action How Unique is Water?? Water is one of only 3 naturally occurring inorganic liquids (mercury and ammonia) Only chemical compound that exists in all 3 states—solid, liquid, and gas Extremely large liquid range (0oC - 100oC) Expands, becomes less dense as a solid Water’s 3 states differ in the degree of H-bonding http://mutuslab.cs.uwindsor.ca/schur ko/animations/waterphases/status_wa ter.htm Liquid water has H-bonds that form and break constantly http://www.stolaf.edu/people/giannini/flashanimat/w ater/water.swf Allows water to have a high specific heat Amount of energy required to raise temp of 1 g 1o C 1 cal/1 g Water Vapor As water moves from liquid to gaseous state, Hbonds are broken, allowing water molecules to escape Water has a high heat of vaporization: It takes 540 cal for 1 g of water to move from liquid to gaseous state Allows for evaporative cooling As fast-moving liquid water molecules escape as vapor, they take their heat energy with them Ice Solid water is less dense than liquid water, allowing it to float H-bonds lock into lattice structure Acids and Bases Water molecules have slight tendency to ionize: H2O < -- > H+ + OH- The H+ then joins another water molecule resulting in H3O+ (hydronium) The pH scale is a measure of hydronium concentration expressed in moles/liter pH scale http://www.johnkyrk.com/H2O.html pH = -log10[H+] Acids are proton donors Increase the # of H+ ions Bases are proton acceptors Acid + Base Salt Acid = H+ + anion Base = OH- + cation H+ joins with OH- to get H2O Anion can combine with cation to make a salt Ex. HCl + NaOH H2O + NaCl Buffers Substance(s) that resist pH changes when an acid or base is added Usu. weak acid or base, do not completely ionize Example: Blood in Vertebrates CO2 + H20 < -- > H2CO3 < -- > H+ + HCO3- Will stay at dynamic equilibrium unless stressed If add excess H+ system shifts left and forms carbonic acid If add OH- they combine with H+ forming water, system shifts right http://www.tvdsb.on.ca/westmin/science/sbioac/biochem/buf fer.htm http://www.mhhe.com/physsci/chemistry/essentialch emistry/flash/buffer12.swf