101Ch8
advertisement
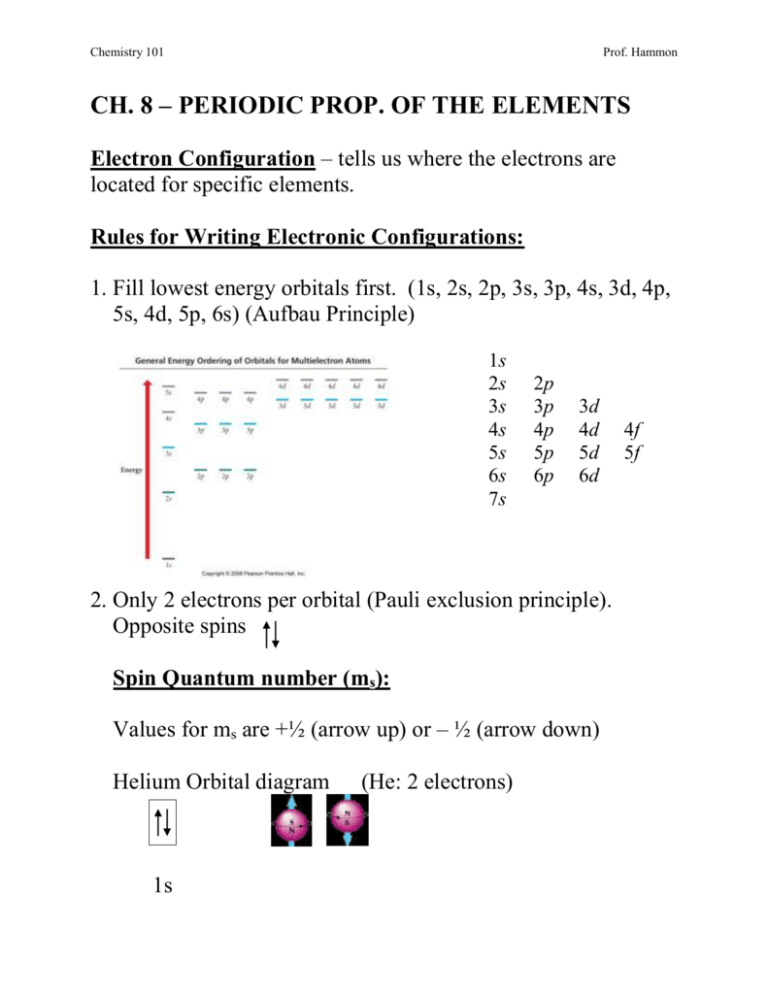
Chemistry 101 Prof. Hammon CH. 8 – PERIODIC PROP. OF THE ELEMENTS Electron Configuration – tells us where the electrons are located for specific elements. Rules for Writing Electronic Configurations: 1. Fill lowest energy orbitals first. (1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s) (Aufbau Principle) 1s 2s 3s 4s 5s 6s 7s 2p 3p 4p 5p 6p 3d 4d 5d 6d 2. Only 2 electrons per orbital (Pauli exclusion principle). Opposite spins Spin Quantum number (ms): Values for ms are +½ (arrow up) or – ½ (arrow down) Helium Orbital diagram 1s (He: 2 electrons) 4f 5f Chemistry 101 Prof. Hammon He electron config.: 1s2 (e- represented by superscript) Quantum #s for 2 electrons in Helium: n 1 1 l 0 0 ml 0 0 ms +½ -½ 3. Don’t pair electrons in equal energy orbitals until you have to (Hund’s Rule). Nitrogen: 7 electrons N Orbital diagram 1s 2s 2px 2py 2pz N electron config.: 1s22s22p3 Example: Write orbital diagram & electron configuration for: Element # of e- e- config. Orbital diagram K Write the set of 4 quant #s that represent the last e- added to K n l ml ms Chemistry 101 Prof. Hammon Noble Gas Shorthand Electron Configurations (Inner Electron Configuration): K Write electron configurations for: F F- K+ Chemistry 101 Prof. Hammon For transition metal ion remove ns electrons before (n-1)d electrons. Fe Fe2+ Zn Zn2+ Paramagnetic: Atom contains unpaired electrons. Attracted to a magnetic field. Diamagnetic: Atom contains paired electrons only. Not attracted to a magnetic field. Is iron paramagnetic or diamagnetic? Is zinc paramagnetic or diamagnetic? Chemistry 101 Prof. Hammon Valence Electrons – electrons in the outer most shell (the one with the highest principle quantum #) Core Electrons – electrons not in the outermost shell i.e. Phosphorus (P) electron configuration 1s22s22p63s23p3 Core e- valence e- Determine the valence and core electrons for calcium. Determine the valence and core electrons for gallium (Ga). Exception: Transition Metals with d orbitals that are not completely filled. The electrons in the outermost d orbitals are counted as valence e-. Determine the valence and core electrons for titanium (Ti). Chemistry 101 Prof. Hammon Electron Configurations & the Periodic Table Elements in a family (column) have the same # of valence electrons. Column # corresponds to # of valence electrons. Row # corresponds to highest shell (principle quantum #). The highest quantum # increases by 1 as move down column. Columns 1A & 2A have s block outer shell electron config. Columns 3A-8A have p block outer shell electron config. Transition metals have d block electron config. Lanthanides & Actinides have f block electron config. To write Electron Configurations from the periodic table: 1) Write core electron configuration as proceeding noble gas in brackets. 2) Write valence electrons by assigning highest principal quantum # as row # & determine # of e- in s, p, d or f blocks. 3) For elements containing d electrons, the principle quantum # is equal to the row # minus 1. 4) For elements containing f electrons, the principle quantum # is equal to the row # minus 2. Chemistry 101 Prof. Hammon Write the electron configuration for selenium using the periodic table. Write the electron configuration for titanium using the periodic table. Write the electron configuration for europium (Eu) using the periodic table. Chemistry 101 Prof. Hammon THE OCTECT RULE Atoms and ions are most stable when they have a complete outer shell of eight electrons. (Noble-Gas wanna-be’s) i.e. Na 1s22s22p63s1 (outer shell has ___e-) Na+ + Na Na+ i.e. e- 1s22s22p6 (outer shell has ___ e-) [Ne] O 1s22s22p4 (outer shell has ___ e-) O + O2- 2e- O2- 1s22s22p6 (outer shell has ___ e-) [Ne] Octet Rule (some reasons why it is not a perfect rule): 1. Hydrogen can only acquire 2 electrons. 2. The octet rule can’t be applied to transition elements because they are too far removed from a noble gas structure. Chemistry 101 Prof. Hammon Goodyear Blimp vs. the Hindenburg • • Hindenburg Hydrogen – Reactive and Flammable Goodyear Blimp Helium – Inert (non-reactive) and Inflammable Periodic Law: When elements are arranged in order of increasing atomic number, certain sets of properties recur periodically. Example: Hydrogen behaves like Li and Na & Helium behaves like Ne and Ar (inert gases). Why is hydrogen more reactive than helium? Chemical properties are mostly determined by valence electrons. Atoms with 8 valence electrons are stable. Alkali metals are reactive with one valence electron (if lose electron will have noble gas electron configuration). Why are halogens reactive? Chemistry 101 Prof. Hammon PERIODIC TRENDS Atomic Size: how far the outermost electrons are from the nucleus determines the size of the atom. Atomic size increases as you move down a family of the periodic table. Atomic size decreases as you move right across a period of the periodic table. Is Ca or Ba larger? Effective Nuclear Charge increases as you move across a row of the periodic table resulting in decrease of atomic size. Z effective (effective nuclear charge): the amount of positive charge an outermost electron experiences. Slater’s Rule Z = nuclear charge of atom (#of protons) S = Shielding: the amount of positive charge blocked by other electrons. Chemistry 101 Prof. Hammon Shielding (S): Actual percent of shielding experienced by outermost electron is dependent on # of e- & location of other electrons. Principle Quantum # same n n-1 n-2 (or lower) Shielding 35% 85% 100% S=[# of e- in same n ___ x (0.35)] + [# of e- in n-1__ x (0.85)] + [# of e- in n-2(or <)__ x (1.00)] Calculate Zeff for Aluminum Al electron config.: S = [# of e- in same n ___ x (0.35)] + [# of e- in n-1___ x (0.85)] + [# of e- in n-2 ___ x (1.00)] S = _____ Z= ____ Zeff = Z – S Chemistry 101 Prof. Hammon Calculate Zeff for Silicon Si electron config.: S= Z= ____ Zeff = Z – S Calculate Zeff for Phosphorous P electron config.: Ion Size Cations are always smaller than the atoms they come from. Anions are always larger than the atoms they come from. Chemistry 101 Prof. Hammon PERIODIC TRENDS Ionization Energy (I.E.): energy required to remove an electron from an atom in the gaseous state. 1st Ionization Energy: energy required to remove 1st electron. 2nd Ionization Energy: energy required to remove 2nd electron. Ionization energy increases as you move to the right across a row (period) of the periodic table. (Zeff increases so harder to remove electrons) Ionization energy decreases as you move down a column (family) of the periodic table. (electrons farther from nucleus are easier to remove) Does Mg or Cl have a higher ionization energy? I.E. Exception: I.E. for oxygen (1300 kJ/mol) is less than nitrogen (1400 kJ/mol). It is easier to remove an electron from oxygen than nitrogen due to electron-electron repulsion. Chemistry 101 Prof. Hammon Electron Affinity (E.A.): energy associated with gaining an electron by an atom in the gaseous state. Electron Affinities become more negative as you move to the right across a row of the periodic table. (adding an electron becomes more exothermic) Electron Affinities become more negative as you move up group 1A metals. (adding an electron becomes more exothermic) Does Li or K have a more negative electron affinity? E.A. Exception: Fluorine in halogen trend for E.A. E.A. is less negative for F (-328 kJ/mol) than Cl (-349 kJ/mol), because electrons are entering a smaller more densely packed F atom that leads to electron–electron repulsion.







