Properties of Water
advertisement

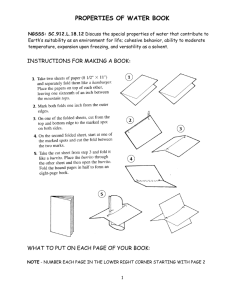
Water Describe Water (46NBtop) How would you describe water to someone who had never seen it before? • You might say that pure water has no color, no taste, and no odor. You might even say that water is a rather plain, ordinary substance. • If you asked a chemist to describe water, the chemist would say that water is very unusual. Its properties differ from those of most other familiar substances. NB Setup: – NB44 - Hydrosphere Unit Page • (label & decorate) – – – – – – NB45 - Hydrosphere Objectives & Tracker NB46 – Describe Water / NB47 - Unique Properties of Water Webquest NB48 - Unique Properties of Water Foldable NB49 – Properties of Water Station Lab NB50 – Frayer Diagrams Cohesion/Adhesion Why is water so Important? • • • • All life on Earth depends on water Our fresh water resources are scarce Global warming is effecting the hydrosphere Different types of pollution are contaminating all of our water • Etc, etc, etc…. • Now…. Let’s look at what makes water so special in the first place…. Properties of Water foldable Instructions • Fold your white paper into a 3-fold brochure Title is “Unique Properties of Water” Label inside 3 section: • • 1. Universal Solvent/Solubility 2. Cohesion/Adhesion (Capillary Action!!) 3. Surface Tension • Label two outside sections: • • Polarity/Specific Heat Density/Buoyancy foldable Instructions What goes in our foldable? Facts! Definitions! Pictures! Be creative! Own it! What seems important TO YOU?? Polarity of Water • In a water molecule two hydrogen atoms form single polar covalent bonds with an oxygen atom. – A water molecule is a polar molecule with opposite ends of the molecule with opposite charges. Water has a variety of unusual properties because of attractions between these polar molecules. – Each water molecule can form hydrogen bonds with up to FOUR neighbors. – Gives water more structure than other liquids… and therefore causes lots of interesting things to happen…… foldable Instructions What goes in our foldable for Polarity? Facts! Definitions! Pictures! Be creative! Own it! What seems important TO YOU?? Capillary Action Now, how does that work?? Hydrogen bonds hold water together, a phenomenon called …. Cohesion & Adhesion Cohesion Co-hesion= 1. “Co-” = share 2. “-hesion”= bonded example -> (adhere) So… Cohesion refers to waters attraction to other water molecules. Adhesion – Adhesion refers to attraction to other substances. • Water is adhesive to any substance with which it can form hydrogen bonds. Trees have specialized structures to transport water: - xylem and phloem “plumbing” * Water molecules are “dragged” from the roots to the top of the tree by capillary action and cohesion! Capillary action water evaporates from leaves = transpiration adhesion, cohesion and capillary action water taken up by roots Surface Tension • Have you ever watched water striders (spiders!!!) skate across the surface of a pond without sinking? • They are supported by the surface tension of the water…. • Surface tension is the tightness across the surface of water that is caused by the polar molecules pulling on one another. (its related to cohesion) Some animals can stand, walk, or run on water without breaking the surface. – Water has a greater surface tension than most other liquids because hydrogen bonds among surface water molecules resist stretching or breaking the surface. – Water behaves as if covered by an invisible film. Where else have you seen surface tension in action? Specific Heat • It is a steamy summer day. The air is hot, the sidewalk is hot, and the sandy beach is hot. But when you jump into the ocean, the water is surprisingly cool! If you go for an evening swim, however, the water is warmer than the cool air. Because…. • Water has a HIGH Specific Heat. • Specific Heat is the amount of heat that must be absorbed or lost for one gram of a substance to change its temperature by 1oC. Three-fourths of the earth is covered by water. The water serves as a large heat sink responsible for: 1. Prevention of temperature The Earth is over 75% water! fluctuations that are outside the range suitable for life. 2. Coastal areas having a mild climate 3. A stable marine environment Universal Solvent “Universal” Solvent • What happens when you make a fruit drink from a powdered mix? • homogeneous = solution. • In an aqueous solution, water is the solvent. Shhh….Water is not really a universal solvent, but it is very versatile because of the polarity of water molecules. Solvent for Life • Can be either: – Solute (if its not main ingredient) – Solvent (= Aqueous solution ) • Hydrophilic (H20 loving) – Ionic compounds dissolve in water – Polar molecules (generally) are water soluble • Hydrophobic (H20 phobic) Nonpolar compounds Cohesion and solubility allow…. • Water to transport molecules dissolved in it: – Blood, a water-based solution, transports molecules of nutrients and wastes organisms – Nutrients dissolved in water get transported through plants – Unicellular organisms that live in water absorb needed dissolved substances Density of Water • Most dense at 4oC • Contracts until 4oC • Expands from 4oC to 0oC The density of water: 1. Prevents water from freezing from the bottom up. 2. Ice forms on the surface first—the freezing of the water releases heat to the water below creating insulation. 3. Makes transition between season less abrupt. Solid water (ice) is less dense than liquid • Ice is less dense than water: the molecules are spread out to their maximum distance Density = mass/volume same mass but a larger volume How does ice float? • Ice is “Buoyant” – It floats on top of the more dense liquid water… – But what is “Buoyancy”? Oceans and lakes don’t freeze solid because ice floats water expands as it solidifies water reaches maximum density at 4-degrees C water freezes from the top down organisms can still live in the water underneath the ice during winter Water is Transparent • The fact that water is clear allows light to pass through it – Aquatic plants can receive sunlight – Light can pass through the eyeball to receptor cells in the back Let’s Wrap it up! • Ticket out the door: – 3/2/1: 3 things you learned, 2 questions, 1 favorite or Least favorite thing about today’s lesson • H.W. - Brochure – Follow given rubric, and use notes from today – Due Mon/Tues Warm-Up - 46NBbottom What properties of water do you feel make it a Unique/Special Substance? Explain and justify your response. Frayer Diagrams Stations on Water Properties Let’s Wrap it up! • Ticket out the door ~ Take #2 – 3/2/1: 3 things you learned, 2 questions, 1 favorite or Least favorite thing about today’s lesson – Psst! (did your questions from last lesson get answered? Let me know!!) • H.W. – Frayer Diagrams completed • Also: – Complete all station reports, if not completed in Class. – Notebooks due Friday for NB check!