Properties of Water - Magoffin County Schools

Properties of Water
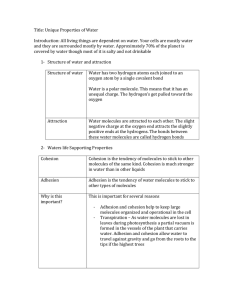
Water is essential to life on Earth. The physical properties of water molecules give the substance several unique characteristics: cohesion, adhesion, and high specific heat. Recall that water is a polar molecule with a partial positive and a partial negative end. The negative end of one water molecule (oxygen) is attracted to the positive end
(hydrogens) of other water molecules. These attractions create hydrogen bonds between the individual molecules of water. One water molecule can have up to four hydrogen bonds with other nearby water molecules. This network of electrostatic associations has special implications for water both as a pure liquid and as a solvent.
Cohesion is the intermolecular attraction between molecules. In water, it results from hydrogen bonding between the water molecules. This creates a highly structured liquid environment. Essentially water molecules are loosely attracted to one another and tend to stick together. This is what gives water its high surface tension. Additionally, cohesive attraction imparted by the hydrogen bonds causes water to spread out as the temperature decreases. This reduces the density as the solid ice crystals form. The result: ice floats. Floating ice means that bodies of water will freeze from the top down, allowing space below the ice for organisms to survive. Cohesion is also the reason water droplets form spheres.
The polarity of water gives it another interesting characteristic called adhesion. Adhesion is the ability of water to stick to other water-loving
(hydrophilic) molecules. This makes water an excellent solvent, especially for polar and ionic solutes. Let’s look at what happens when water dissolves table salt (NaCl). Remember, table salt is an ionic solid bound in a crystalline state. When the salt is added to water, the charged ends of the water molecule are attracted to the opposite charge on the ionic solid. Remember form physical science-opposite charges attract. Their electrostatic attraction is stronger than the chemical bonds holding the salt together. This breaks the bonds of the ionic salt and causes it to dissolve into the solvent. Now the individual sodium
(Na + ) and chlorine (Cl ) ions are available to living things.
Taken together, the adhesive and cohesive properties of water allow plants to move water vertically, sometimes several hundred feet in the air! These properties of water also help move substances like foods and ions throughout the organism.
Specific heat refers to the amount of energy it takes to change one gram of water one degree Celsius. Due to its hydrogen-bound structure, water has a high specific heat. This means that it takes a lot of energy to destabilize the hydrogen bonds of water and thus change it temperature. This resistance to abrupt changes in temperature shields organisms from harsh temperature extremes. It also means that water often is at a different temperature than the ambient air. Because of this temperature differential water can cool or warm surrounding air masses and be the source of changing weather conditions.
Review Questions
1.
Define the following terms: a.
Cohesion b.
Adhesion c.
Specific heat
2.
Aluminum chloride is an ionic molecule that makes up the polar molecules of AlCl3. Will this molecule dissolve in water? a.
No b.
Yes c.
No, aluminum should never dissolve in aqueous solution d.
Yes, but it will happen so slowly that no one will notice
3.
A water strider is a type of insect that “walks” on the surface of the water without getting its feet wet. What property of water is this insect using to stay on the water’s surface? a.
Specific heat b.
Adhesion c.
Cohesion d.
Adhesion and cohesion
4.
Kiko dipped the corner of a paper towel into a glass of water.
While she knows that gravity is pulling down on the molecules of water, she still observes water moving vertically up the sheet of paper towel. What property of water caused this to occur? a.
Specific heat
b.
Adhesion c.
Cohesion d.
Adhesion and specific heat
5.
What is the main reason the polarity of water is important to living things?
6.
Why is water called the universal solvent?
7.
Why is floating ice important to living things?