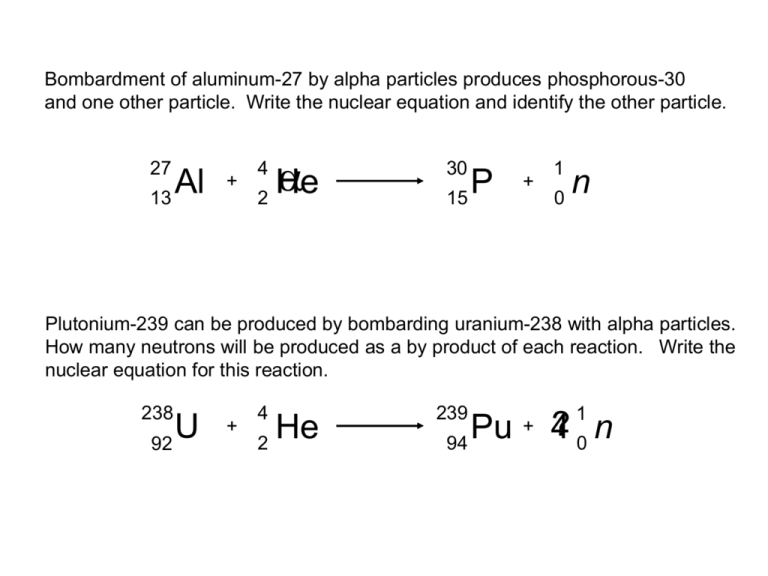
Bombardment of aluminum-27 by alpha particles produces phosphorous-30
and one other particle. Write the nuclear equation and identify the other particle.
27
13
Al
+
a
He
2
4
30
15
P
+
1
0
n
Plutonium-239 can be produced by bombarding uranium-238 with alpha particles.
How many neutrons will be produced as a by product of each reaction. Write the
nuclear equation for this reaction.
238
92
U
+
4
2
He
239
94
Pu
+
1
?0n
4
Unstable Isotopes
and
+
or
Excited
nucleus
Kelter, Carr, Scott, Chemistry A World of Choices 1999, page 439
Stable
nucleus
Energy
Particles
Radiation
Unstable Nucleus
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 620
Fissionable U-235
Fission Process
Nucleus
Neutron
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 620
Two neutrons
from fission
Stages of Fission
First stage: 1 fission
Kelter, Carr, Scott, Chemistry A World of Choices 1999, page 454
Second stage: 2 fissions
Third stage: 4 fissions
Nuclear Power Plants
map: Nuclear Energy Institute
Energy Sources in the United States
100
91
Percent
80
71
70
60
50
40
40
20
58
50
21
9
26
20
5
10
3
21
26
16
10
0
1850
Wood
1900
Coal
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 307
1940
1980
Petroleum / natural gas
1990
2005
Hydro and nuclear
Energy Sources in the United States
100
91
Percent
80
60
50
40
20
19 19
9
7
3
3
0
2005
1850
Coal
Petroleum
Nuclear
Hydroelectric
natural gas
Renewable
(biomass, geothermal, solar, wind)
Source: US Energy Information Administration (2005 Electricity Generation)
Coal Burning Power Plant
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Nuclear Power Plant
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 621
Reactor Core
Hot coolant
Control rods of
neutron-absorbing
substance
Uranium in fuel
cylinders
Incoming coolant
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 622
Nuclear Power Plant
Production of heat
Copyright © 2006 Pearson Benjamin Cummings. All rights reserved.
Production of electricity
Chant of the Radioactive Workers
We're not afraid of the alpha ray.
A sheet of paper will keep it away!
A beta ray needs much more care,
Place sheets of metal here and there.
And as for the powerful gamma ray
(Pay careful heed to what we say)
Unless you wish to spend weeks in bed
Take cover behind thick slabs of lead!
Fast neutrons pass through everything.
Wax slabs remove their nasty sting.
These slow them down, and even a moron
Knows they can be absorbed by boron.
Remember, remember all that we've said,
Because it's no use remembering when you're dead.
Inside a nuclear power plant.
Shaft
Surface
deposits
Nuclear Waste
Disposal
Aquifier
River
Interbed
rock layer
Host rock
formation
Repository
Waste
package
Interbed
rock layer
Aquifier
Bedrock
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 626
Waste
form
Half-Life
20 g
10 g
5g
Start
after
1 half-life
Dorin, Demmin, Gabel, Chemistry The Study of Matter 3rd Edition, page 757
after
2 half-lives
2.5 g
after
3 half-lives
b emissions
131
53
I
89.9%
7.3%
Half-Life
0.500 mg
1.00 mg
131
53
I
0.750 mg
Xe
0.875 mg
0.500 mg
131
53
0.00 days
I
0.250 mg
8.02 days
131
I
53
Dorin, Demmin, Gabel, Chemistry The Study of Matter 3rd Edition, page 757
131
Xe
54
0.125 mg
24.06 days
16.04 days
+
Xe*
g emissions
131
54
131
54
131
54
b
-1
0
+
g
Xe
Half-life of Radiation
Radioisotope remaining (%)
Initial amount
of radioisotope
100
After 1 half-life
After 2 half-lives
50
After 3 half-lives
t1/2
25
t1/2
12.5
t1/2
0
1
2
3
Number of half-lives
4
Half-Life Plot
Amount of Iodine-131 (g)
20
Half-life of iodine-131 is 8 days
15
1 half-life
10
2 half-lives
5
3 half-lives
4 half-lives
etc…
0
0
8
16
24
Time (days)
Timberlake, Chemistry 7th Edition, page 104
32
40
48
56
Half-Life of Isotopes
Half-Life and Radiation of Some Naturally Occurring Radioisotopes
Isotope
Half-Live
Radiation emitted
Carbon-14
5.73 x 103 years
b
Potassium-40
1.25 x 109 years
b, g
Radon-222
3.8 days
a
Radium-226
1.6 x 103 years
a, g
Thorium-230
7.54 x 104 years
a, g
Thorium-234
24.1 days
b, g
Uranium-235
7.0 x 108 years
a, g
Uranium-238
4.46 x 109 years
a
Half-life (t½) /
1
Calcium
Ratio of Remaining Potassium-40 Atoms
to Original Potassium-40 Atoms
Argon
1/
4
1/
8
1/
16
– Time required for half the atoms of a
radioactive nuclide to decay.
– Shorter half-life = less stable.
1/1
Potassium
2
Newly formed
rock
1/2
1/4
1/8
1/16
0
0
1 half-life
1.3
2 half-lives
2.6
3 half-lives
3.9
Time (billions of years)
4 half-lives
5.2
Half-life (t½)
Potassium
Argon
Calcium
Ratio of Remaining Potassium-40 Atoms
to Original Potassium-40 Atoms
– Time required for half the atoms of a
radioactive nuclide to decay.
– Shorter half-life = less stable.
1/1
Newly formed
rock
1/2
1/4
1/8
1/16
0
0
1 half-life
1.3
2 half-lives
2.6
3 half-lives
3.9
Time (billions of years)
4 half-lives
5.2
How Much Remains?
After one half-life,
1
2
of the original atoms remain.
After two half-lives, ½ x ½ = 1/(22) = 1 4 of the original atoms remain.
After three half-life, ½ x ½ x ½ = 1/(23) = 1 8 of the original atoms remain.
After four half-life, ½ x ½ x ½ x ½ = 1/(24) = 1 16 of the original atoms remain.
After five half-life, ½ x ½ x ½ x ½ x ½ = 1/(25) =
1
32
of the original atoms remain.
After six half-life, ½ x ½ x ½ x ½ x ½ x ½ = 1/(26) = 1 64 of the original atoms remain.
1
2
Surviving
“parent”
isotopes
Beginning
1 half-life
Accumulating
“daughter”
isotopes
1
4
1
8
2 half-lives
3 half-lives
1
16
4 half-lives
1
32
5 half-lives
1
64
6 half-lives
1
128
7 half-lives
1. A small piece of
fossil is burned in
a special furnace.
2. The burning creates carbon
dioxide gas comprised of carbon-12
isotopes and carbon-14 isotopes.
Nitrogen
Stable
C-12 isotope
Decaying
C-14 isotope
3. As the carbon14 decays into
nitrogen-14, it
emits an electron.
4. A radiation
counter records
the number of
electrons emitted.
Note: Not to scale.
SOURCE: Collaboration for NDT Education
MATT PERRY / Union-Tribune
Electron
The iodine-131 nuclide has a half-life of 8 days. If you originally have a
625-g sample, after 2 months you will have approximately?
a.
b.
c.
d.
e.
40 g
20 g
10 g
5g
less than 1 g
N = No(1/2)n
N = amount remaining
No = original amount
n = # of half-lives
N = (625 g)(1/2)7.5
N = 3.45 g
Data Table: Half-life Decay
~ Amount
625 g
312 g
156 g
78 g
39 g
20 g
10 g
5g
2.5 g
1.25 g
Time
0d
8d
16 d
24 d
32 d
40 d
48 d
56 d
64 d
72 d
# Half-Life
0
1
2
3
4
5
6
7
8
9
Assume 30 days = 1 month
60 days
= 7.5 half-lives
8 days
Given that the half-life of carbon-14 is 5730 years, consider a
sample of fossilized wood that, when alive, would have contained
24 g of carbon-14. It now contains 1.5 g of carbon-14.
How old is the sample?
Data Table: Half-life Decay
ln N = - k t
No
t1/2
=
5730 y =
ln 2
0.693
k
Amount
Time
24 g
12 g
6g
3g
1.5 g
0y
5,730 y
11,460 y
17,190 y
22,920 y
# Half-Life
0
1
2
3
4
0.693
k
k = 1.209 x 10-4
ln 1.5 g = - (1.209x10-4) t
24 g
t = 22,933 years
Half-Life Practice Calculations
•
The half-life of carbon-14 is 5730 years. If a sample originally contained
3.36 g of C-14, how much is present after 22,920 years?
0.21 g C-14
•
Gold-191 has a half-life of 12.4 hours. After one day and 13.2 hours, 10.6 g
of gold-19 remains in a sample. How much gold-191 was originally present
in the sample?
84.8 g Au-191
There are 3.29 g of iodine-126 remaining in a sample originally containing
26.3 g of iodine-126. The half-life of iodine-126 is 13 days. How old is the
sample?
39 days old
•
•
A sample that originally contained 2.5 g of rubidium-87 now contains 1.25 g.
The half-life of rubidium-87 is 6 x 1010 years. How old is the sample? Is this
possible? Why or why not?
6 x 1010 years
(60,000,000,000 billions years old)
What is the age of Earth???
Demo: Try to cut a string in half seven times (if it begins your arm’s length).
The half-life of carbon-14 is 5730 years. If a sample originally contained
3.36 g of C-14, how much is present after 22,920 years?
Data Table: Half-life Decay
t1/2 = 5730 years
n =
Amount
22,920 years
5,730 years
3.36 g
0y
1.68 g 5,730 y
0.84 g 11,460 y
0.42 g 17,190 y
0.21 g 22,920 y
n = 4 half-lives
(# of half-lives)(half-life) = age of sample
(4 half-lives)(5730 years) = age of sample
22,920 years
Time
# Half-Life
0
1
2
3
4
Uranium Radioactive Decay
238
234
230
Mass number
226
222
a
4.5 x 109 y
24 d
1.2 m
2.5 x 105 y
8.0 x 104 y
1600 y
3.8 d
3.0 m
27 m
160 ms
5.0 d
138 d
stable
Th-234
b
a
U-238
Pa-234 b U-234
a
Th-230
Ra-226
a
Rn-222
a
218
Po-218
a
214
Pb-214
b
210
Pb-210
206
Pb-206
b
81
82
Bi-214
b
Po-214
a
Bi-210
b
Po-210
a
83
84
85
86
87
Atomic number
88
89
90
91
92
140
Nuclear
Stability
130
120
110
100
Decay will occur in
such a way as to
return a nucleus to
the band (line) of
stability.
Neutrons (N)
90
80
70
60
50
40
30
20
10
0
10
20
30
40
50
Protons (Z)
60
70
80
90
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
160
150
Band of
Stability
140
130
120
Number of neutrons
110
n= p
100
90
80
70
60
50
40
Stable nuclides
Naturally occurring radioactive nuclides
Other known nuclides
30
20
10
0
10
20
30
40
50
60
70
80
Number of protons
90
100 110
140
a decay
209
83 Bi
N
1.52
Z
130
120
184
74 W
N
1.49
Z
110
100
Neutrons (N)
90
b decay
80
107
47 Ag
70
N
1.28
Z
60
50
N
1
Z
56
26 Fe
40
N
1.15
Z
30
positron emission and/or
electron capture
20
20
10 Ne
N
1.0
Z
10
0
10
20
30
40
50
Protons (Z)
60
70
80
90
140
a decay
209
83
Bi
130
N
1.52
Z
120
184
74
W
110
N
1.49
Z
Nuclear
Stability
100
90
Decay will occur in
such a way as to
return a nucleus to
the band (line) of
stability.
Neutrons (N)
b decay
80
107
47
Ag
70
N
1.28
Z
60
50
N
1
Z
56
26
Fe
40
N
1.15
Z
30
positron emission and/or
electron capture
20
20
10
Ne
10
N
1.0
Z
0
10
20
30
40
50
Protons (Z)
60
70
80
90
Half-Lives of Some Isotopes of Carbon
Nuclide
Half-Life
Carbon-9
Carbon-10
Carbon-11
Carbon-12
Carbon-13
Carbon-14
Carbon-15
Carbon-16
0.127 s
19.3 s
10.3 m
Stable
Stable
5715 y
2.45 s
0.75 s
Enlargement of part of band of stability around Neon
23
10
23
10
Ne moves into band of
22
10
stability by beta decay.
23
10 Ne
19
10
0
-1b
by positron emission. Electron
capture would also move 199 F
into the band of stability.
10b
Ne
23
11Na
21
10
Ne
Ne moves into band of stability
19
10 Ne
Ne
19
9
F
20
10
Ne
19
9F
19
10
Umland and Bellama, General Chemistry 2nd Edition, page 773
Ne
23
11
Na
Number of protons
Effects of Radioactive Emissions
on Proton and Neutrons
Loss of 4 He
2
Loss of 0 e
-1
Loss of 0 e or
1
electron capture
Number of protons
Nuclear Decay
“absorption”, “bombardment” vs. “production”, “emission”
223
88
4
2
a
2+
87
37
4
2
Ra
14
7
+
a
2+
17
8
N
0
-1 b
Rb
219
86
+
O +
87
38
+
Alpha
4
2
a
1
1
Sr
Beta
2+
0
-1 b
neutron
1
0
n
2
1
Rn
H
H
2
1
+
H
14
6
+
3
1
2
1
4
2
H
4
2
H
0
-1 b
C
Positron
0
+1
+
He +
He
17
7
N
Gamma
b
0
0
proton
1
1
H
1+
g
1
0
n
Units Used in Measurement
of Radioactivity
Units
Measurements
Curie (C)
radioactive decay
Becquerel (Bq)
radioactive decay
Roentgens (R)
exposure to ionizing radiation
Rad (rad)
energy absorption caused by ionizing radiation
Rem (rem)
biological effect of the absorbed dose in humans
Effects of Instantaneous Whole-Body
Radiation Doses on People
Dose, Sv (rem)
Effect
Alexander Litvinenko
>10 (1000)
Death within 24 h from destruction of the neurological
system.
7.5 (750)
Death within 4-30 d from gastrointestinal bleeding.
1.5 – 7.5 (150 – 750)
Intensive hospital care required for survival. At the
higher end of range, death through infection resulting
from destruction of white-blood cell-forming organs
usually takes place 4 – 8 weeks after accident.
Those surviving this period usually recover.
< 0.5 (50)
Only proven effect is decrease in white blood cell count.
The intensity of radiation is
proportional to 1/d2, where d is the
distance from the source.
Alpha, Beta, Positron Emission
Examples of Nuclear Decay Processes
b- emission
(beta)
a emission
(alpha)
238
92
U 42 He
234
90
Th 42 He
226
88
Ra 42 He
222
86
230
90
226
88
Th
Ra
Rn
27
12
Mg -01e
14
8
O 01e
14
7
Cl
32
17
Cl 01e
32
16
Ca
14
8
O 01e
14
7
27
13
S -01e
35
17
K -01e
40
20
35
16
40
19
b emission
(positron)
Al
N
S
N
Although beta emission involves electrons, those electrons come from the nucleus. Within the nucleus,
a neutron decays into a proton and an electron. The electron is emitted, leaving behind a proton to
replace the neutron, thus transforming the element. (A neutrino is also produced and emitted in the process.)
Herron, Frank, Sarquis, Sarquis, Schrader, Kulka, Chemistry, Heath Publishing,1996, page 275
Nuclear Reactions
First recognized natural transmutation of an element (Rutherford and Soddy, 1902)
226
88
Ra α
4
2
222
86
Rn
First artificial transmutation of an element (Rutherford, 1919)
14
7
N a
4
2
O
?
17
8
1
1
p
Discovery of the neutron (Chadwick, 1932)
9
4
Be a
4
2
12
6
C ?n
1
0
Discovery of nuclear fission (Otto Hahn and Fritz Strassman, 1939)
235
92
U n
Bailar, Chemistry, pg 361
1
0
Ba
141
56
92
36
Kr 3 n
1
0
Preparation of Transuranium Elements
Atomic
Number
Name
Year
Symbol Discovered
93
Neptunium
Np
94
Plutonium
Pu
1940
1940
Reaction
1
U
n
92
0
238
2
U
H
92
1
238
Np
93
238
Americium
Am
1944
239
96
Curium
Cm
1945
239
97
Berkelium
Bk
1949
241
Cf
Ralph A. Burns, Fundamentals of Chemistry 1999, page 553
1950
0
Pu
e
94
-1
238
0
Am
e
95
-1
240
4
Pu
He
94
2
4
Am
He
95
2
4
Cm
He
96
2
242
Californium
1
Np
2
n
93
0
238
1
Pu
n
94
0
95
98
0
Np
e
93
-1
239
1
Cm
n
96
0
242
1
Bk
2
n
97
0
243
1
Cf
n
98
0
245
Preparation of Transuranium Elements
Atomic
Number
Name
Year
Symbol Discovered
93
Neptunium
Np
94
Plutonium
Pu
1940
1940
Reaction
1
U
n
92
0
238
2
U
H
92
1
238
Np
93
238
Americium
Am
1944
239
96
Curium
Cm
1945
239
97
Berkelium
Bk
1949
241
Cf
Ralph A. Burns, Fundamentals of Chemistry 1999, page 553
1950
0
Pu
e
94
-1
238
0
Am
e
95
-1
240
4
Pu
He
94
2
4
Am
He
95
2
4
Cm
He
96
2
242
Californium
1
Np
2
n
93
0
238
1
Pu
n
94
0
95
98
0
Np
e
93
-1
239
1
Cm
n
96
0
242
1
Bk
2
n
97
0
243
1
Cf
n
98
0
245
Additional Transuranium Elements
99
100
101
102
103
104
105
106
107
108
109
110
111
112
114
116
118
Einsteinium
Fermium
Mendelevium
Nobelium
Lawrencium
Rutherfordium
Dubnium
Seaborgium
Bohrium
Hassium
Meitnerium
Darmstadtium
Unununium
Ununbium
Es
Fm
Md
Nb
Lr
Rf
Db
Sg
Bh
Hs
Mt
Ds
Uun
Uub
Uuq
1952
1952
1955
1958
1961
1964
1970
1974
1981
1984
1988
1994
1994
1996
1999
2002
2006
(Russia)
(Russia)









