Acids and Bases
advertisement

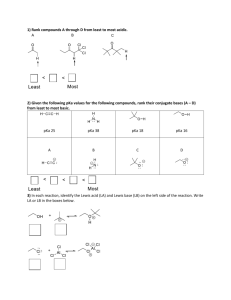
IB Chemistry Power Points Topic 18 Acids and Bases www.pedagogics.ca Buffers and Salts Buffer Solutions DEFINITION: A buffer solution contains a weak acid mixed with its conjugate base (or weak base and conjugate acid) Buffers resist changes in pH when a small amount of a strong acid or base is added to it. HA ∏ H+ + A- If a small amount of a strong acid (H+) is added eqm shifts to the left as [H+] increases so system adjusts to increase [HA] and reduce [H+] again. HA ∏ H+ + A- A small amount of a strong base will react with H+ to form H2O and eqm will shift to the right to increase [H+] again. HA ∏ H+ + A- Making Buffer Solutions An example of a weak acid is ethanoic acid. This could be mixed with sodium ethanoate which will provide ethanoate ions (conjugate base). CH3COOH(aq) ∏ CH3COO-(aq)+H+(aq) An example of a weak base is ammonia. This could be mixed with ammonium chloride to provide ammonium ions (conjugate acid). NH3(aq) + H2O(aq) ∏ NH4+(aq) +OH-(aq) In order for a buffer to work well the concentration of the acid/base and its salt must be much higher than the strong acid/base added. Optimum Buffer A buffer is most effective when the concentration of weak acid and its salt (the conjugate base) are equal and the pH is equal to pKa. In practice it will work reasonably well with similar concentrations and the effective buffer range of any weak acid/ base is pKa 1. Blood has buffering capacity Blood must maintain a pH of 7.4 so its enzymes can work. If 0.01 mol of H+ or OH- is added to blood it only changes pH by 0.1 unit. The eqm is: CO2(aq) + H2O(l) ) ∏ H+(aq) + HCO3-(aq) Buffer Calculation #1 Calculate the pH of a 1.00 dm3 buffer solution made by dissolving 0.50 mol of sodium ethanoate into a 0.075 mol dm-3 ethanoic acid solution. pH = 5.6 Identify the weak acidthe / conjugate OR weak base Write In thisthe case, equilibrium assume equation equilibrium andbase expression concentrations of /the conjugate Determine concentrations. weak acidacid and pair. the salt anion aretheir the same as the given CH3COOH ∏ H+ + CH3COO information (very little change when equilibrium is -3 weakestablished. acid [CH3COOH] = 0.075 mol dm 1. 2.3. [ H ][0.50] 4.76 conjugate base [CH3COO-] = 0.50 mol dm-3 ka [ H ][CH COO 10 ] 3 ka [0.075] 10 pka [CH 3COOH ] 4.76 0.075 10 pH log[ H ] log 0.50 Buffer Calculation #2 Calculate the mass of ammonium chloride that would need to be dissolved into 1.00 dm3 of 0.100 mol dm-3 NH3 solution to create a buffer with a pH of 9.00. Assume no change in overall volume. mass = 9.50 g In thisthe Identify Write case, the equilibrium weak assume acid the equation / conjugate equilibrium andbase expression concentrations OR weak base of the / conjugate weak base acid is thepair. sameDetermine as the given their information concentrations. (very little NH3+ H2Ois∏established. NH4+ + OH-Calculate [OH-] change when equilibrium weakfrom basedesired pH [NH3] = 0.100 mol dm-3 1. 2. 3. conjugate acid [NH4+] = ? (14 pH ) [ NH 4 ][10 ] ] 4.75 [ NH ][ OH 4 kb 10 10 pkb kb[0.100] [ NH 3 ] 4.75 0.100 10 -3 [ NH 4 ] 0.1778 mol dm 10 (149) Buffer Calculation #3 A buffer can also be made by mixing excess weak acid/base with a lesser amount of strong base/acid. For example, calculate the pH of a buffer formed when 25 cm3 of 0.075 mol dm-3 HCl is added to 40 cm3 of a 0.150 mol dm-3 ammonia solution. 1. In 2. 3. Dothis Write thethe case, stoichiometry equilibrium assume the to equation equilibrium determine and what expression concentrations the concentrations of the are AFTER weak base neutralization. and cation are the same as determined by + NH3+ H2Ochange ∏ NHwhen stoichiometry (very little equilibrium is 4 + OH established. HCl + NH3 NH4+ + Cl- pH= 9.6 [0.02885][ OH ] (0.025 0.075) NH 4 kb HCl 104.75 limiting (why?) 0.065 [0.06346] [ NH 4 ][OH ] pkb k 10 NH 4 b0.02885 0.06346 10 4.75 [ NH 3 ] pOH log (0.040 0.150) (0.025 0.075) 0.02885 NH 3 0.065 NH 3 0.06346 Salt Hydrolysis A soluble salt is an ionic compound made of cations (ex. Na+) and anions (ex. Cl-) which completely dissociates into ions in aqueous solution. Salts can affect the pH of a solution because the cations act as weak acids by bonding with OHand the anions act as weak bases by accepting H+ ions. Salt Hydrolysis If the cation comes from a strong base then it will have less acidic activity than one from a weak base. For example Na+ from NaOH is a weaker acid than NH4+ from NH3 If the anion comes from a strong acid then it will have less basic activity than one from a weak acid. For example Cl- from HCl is a weaker base than CH3COO- from CH3COOH. Salts from a strong acid and strong base ex. NaCl will form a neutral solution. Salt Hydrolysis Would a solution of sodium ethanoate be acidic, basic or neutral? Would a solution of ammonium chloride be acidic, basic or neutral? Would a solution of potassium chloride be acidic, basic or neutral? Salt Hydrolysis With salts of weak acids and weak bases the pH of the solution formed will reflect the relative strengths of the acid and base. Ex. Ammonium ethanoate is about neutral. BE CAREFUL THOUGH . . . Acidity of salts also depends on size and charge of the cation Salt Hydrolysis Salts with small, highly charged cations are more acidic than large, low charge cations. recall Period 3 chloride salts : NaCl, MgCl2 and AlCl3 which is most acidic The aluminum ion and those of transition metals exist in water in hydrated form ie. [Al(H2O)6]3+ , [Fe(H2O)6]3+ Salt Hydrolysis the e- attracting power of the ion weakens the O-H bond and stabilizes the resulting OH- ion. As a result these ions are quite acidic in water. [Fe(H2O)6]3+ [Fe(OH)(H2O)5]2+(aq) + H+(aq) Learning Check – salt hydrolysis Acid Base Example Salt Strong Strong Weak Weak ammonium ethanoate neutral Strong Weak ammonium chloride acidic Weak Strong sodium ethanoate basic NaCl Solution neutral IB Chemistry Power Points Topic 18 Acids and Bases www.pedagogics.ca Buffers and Salts Acid Base Titrations If a strong acid is added to a strong base gradually the pH will start off as 1. Once enough base is added that it is now in excess the pH will change very suddenly to about 13. The point at which this change is seen is when the amount of acid = amount of base. This is called the equivalence point. With this combination it occurs at pH 7 as the acid and base combine to make a neutral solution. Strong Acid and Strong Base Most indicators will work for this combination. Weak Acid + Strong Base A weak acid will have a pH of 3-5. When a strong base is added the pH will increase gradually as HA is converted to A-. This is called the buffering region as it’s acting like a buffer. When half the amount of base has been added it is called the half-neutralization point and [HA] = [A-] so [H+] = Ka x [HA]/[A-] Ka = [H+] and pKa = pH Weak Acid + Strong Base Ka = [H+] and pKa = pH This is the best way to determine the Ka for a weak acid. At the equivalence point the pH increases quickly to 13. The equivalence occurs when pH >7. Most suitable indicator is phenolphthalein Strong Acid + Weak Base When a weak base is added to a strong acid the pH will remain around 1 until near the equivalence point when all the base has been converted into its conjugate acid. B(aq) + H+(aq) BH+(aq) At the equivalence point pH is <7 and exact pH can be found from Kb of the base. [OH-] = Kb x [B]/[BH+] At equivalence point [B] = [BH+] so Kb = [OH-] And pKb = pOH = 14 - pH Strong Acid + Weak Base A suitable indicator here is methyl orange. Weak Acid + Weak Base When a weak acid is added to a weak base the change in pH is gradual from acidic to basic so it is hard to detect the equivalence point. It is hard to find a suitable indicator. The volume of base that will neutralize an acid is not affected by the strength. It only depends on the stoichiometric amounts reacting. Learning Check Do exercise 18.4 on packet. Indicators An indicator is a substance (often an organic dye) that has a different color in acidic and alkaline solutions so it can be used to show the end point of a titration. The color change is seen because the indicator is a weak acid/base in which the two forms have different colors. Ex. HIn(aq)H+(aq) + In-(aq) litmus: red blue Hin stands for the indicator and In- is the other form when it dissociates. Each of these forms will have a different color. Indicators In the presence of an acid the eqm is driven to the left to form HIn as the H+ ions combine with In- to reduce [H+]. With a base it shifts to the right to form more In- as the OHof the base combines with H+ reducing [H+] so more Hin will dissociate. The usual equation for Ka applies: Ka = [H+][In-] / [HIn] [HIn] / [In-] = [H+] / Ka Indicators The color depends on the pH and [H+] and also on Ka so different indicators change color over different pH ranges. When pH = pKa the two colored forms will have equal concentrations and the indicator will be in the middle of its color change. If one form is in excess by 10 fold then the color will be of that form. pH of Indicator If [HIn] is 10x [In-] the pH will be: [H+] = Ka x [Hin] / [In-] = Ka x 10/1 = 10Ka or pH = pKa -1 If [In-] were 10x [HIn] then pH = pKa + 1. Many indicators change over a region of 2 pH units. An effective indicator will change color at the equivalence point of the particular titration. Choosing an Indicator Phenolphthalein pKa = 9.6 pH range: 8.3 -10.0 Color in acid: colorless Color in alkali: pink Good for titrations with strong bases Choosing an Indicator Methyl Orange pKa = 3.7 pH range: 3.1 - 4.4 Color in acid: red Color in alkali: yellow Good for titrations with strong acids Learning Check Do exercise 18.5 in packet