Honors Chem- Buffers Worksheet
advertisement

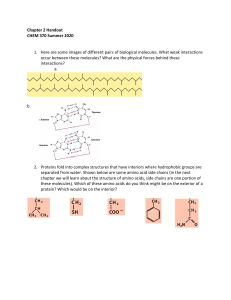
Name __________________ Honors Chem- Buffers Worksheet 1. What two ingredients does a buffer contain? 2. Describe how a buffer controls pH 3. List three weak acids and their conjugate bases. a. b. c. 4. Write the equilibrium reaction for an acetic acid buffer solution. 5. A buffer is prepared containing 1.00 molar acetic acid and 1.00 molar sodium acetate. What is its pH? Ka = 1.76 x 10-5 6. Calculate the pH of a buffer solution made from equal amounts of 0.30 M hydrofluoric acid and 0.70 M sodium fluoride. K a =7.1×10 −4 7. A buffer is prepared with .5 M HCN and the same concentration of its conjugate base. What is the pH of the bufer?