10-3 Integration and the N+1 Rule PPT
advertisement

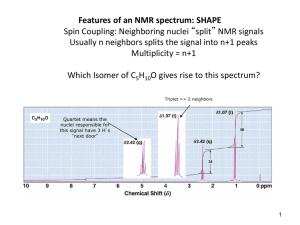
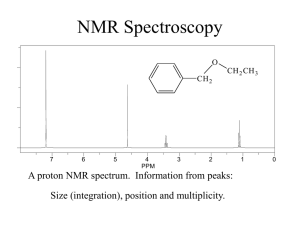
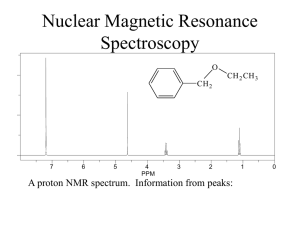
10-6 Integration Integration reveals the number of hydrogens responsible for an NMR peak. The area under an NMR peak is proportional to the number of equivalent nuclei contributing to the peak. By comparing peak areas, it is possible to quantitatively estimate the relative numbers of contributing protons. The areas are obtained by the controlling computer and plotted on top of the regular spectrum by choosing integration mode. Chemical shifts and peak integration can be used to determine structure. Consider the monochlorination of 1-chloropropane: NMR spectroscopy distinguishes all three isomers: 1,1-Dichloropropane: Three NMR signals in the ratio of 3:2:1. Δ = 5.93 ppm (CH), 2.34 pm (CH2), and 1.01 (CH3). 1,2-Dichloropropane: Three NMR signals in the ratio of 3:2:1. δ = 4.17 ppm (CH), 3.68 ppm (CH2), and 1.70 ppm (CH3). 1,3-Dichloropropane: Two NMR signals in the ratio of 2:1. (CH2Cl) and 2.25 ppm (CH2). δ = 3.71 ppm 10-7 Spin-Spin Coupling: The Effect of Non-Equivalent Neighboring Hydrogens When non-equivalent hydrogen atoms are not separated by at least one carbon or oxygen atom, an additional phenomenon called “spin-spin splitting” or “spin-spin coupling” occurs. Instead of single peaks (singlets), more complex patterns occur called multiplets (doublets, triplets or quartets). The number and kind of hydrogen atoms directly adjacent to the absorbing nuclei can be deduced from the multiplicity of the peak. One neighbor splits the signal of a resonating nucleus into a doublet. Consider two protons, Ha and Hb. The population of each of these protons is very close to 50% and 50% in the external magnetic field, H0. This means that in 50% of the molecules, Ha protons will have Hb protons in the state and in 50% of the molecules, Ha protons will have Hb protons in the state. The total field seen by 50% of the Ha protons will therefore be slightly greater than H0 and slightly less than H0 for the other 50 % of the Ha protons. What would have been a singlet NMR peak is now split into a doublet of peaks, symmetrically displaced from the original peak. The chemical shift of the Ha nucleus is reported as the center of the doublet. The amount of mutual splitting is equal. The distance between the individual peaks making up the doublet is called the “coupling constant” (J). Here J is 7 Hz. Coupling constants are independent of the field strength of the NMR spectrometer being used. Spin-spin splitting is usually observed only between hydrogen atoms bound to the same carbon (geminal coupling) or to adjacent carbons (vicinal coupling). Hydrogen nuclei separated by more than two carbon atoms usually negligible. (1,3 coupling) is Finally, equivalent nuclei do not exhibit mutual spin-spin splitting. Ethane exhibits only a single line at δ = 0.85 ppm. Splitting is observed only between nuclei with different chemical shifts. Local-field contributions from more than one hydrogen are additive. Consider the triplet above. It corresponds to the methyl protons being split by the methylene protons. The methylene proton spins will statistically orient in the external magnetic field as , , and . Each methyl proton will see an increased field 25% of the time (), no change 50% of the time ( and ), and a decreased field 25% of the time (). The integrated intensity of the triplet will be 6 since there are a total of 6 equivalent methyl protons. In the case of the methylene protons, the methyl proton spins will statistically distribute as , , , , , , , and . This will result in a 1:3:3:1 quartet of peaks. The integrated intensity of the quartet will be 4, corresponding to the 4 equivalent methylene protons. In many cases, spin-spin splitting is given by the N+1 rule. A simple set of rules: Equivalent nuclei located adjacent to one neighboring hydrogen resonate as a doublet. Equivalent nuclei located adjacent to two hydrogens of a second set of equivalent nuclei resonate as a triplet. Equivalent nuclei located adjacent to a set of three equivalent hydrogens resonate as a quartet. This table illustrates the N+1 rule: Nuclei having N adjacent equivalent neighbors split into N+1 peaks. The heights of the N+1 peaks follow Pascal’s triangle. It is important to note that non-equivalent nuclei split each other. A split in one requires a split in the other. In addition, the coupling constants will be the same for each type of nuclei. Two additional examples: