Long-Range Coupling
advertisement

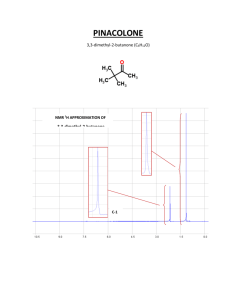
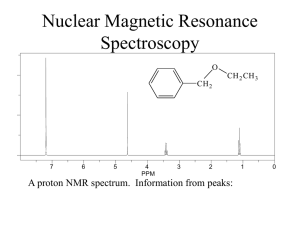
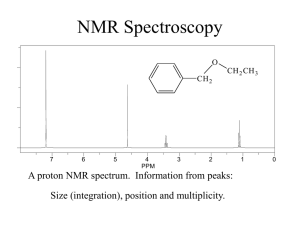
Lecture 9 Aromatics: Long-Range Coupling H’s on aromatic rings may couple with non-neighboring protons due to long-range coupling. You will see this in lab! Why? Nuclei “communicate” via bonding electrons - p electrons that are in resonance will allow non-neighboring H’s to “communicate” and couple/split. This leads to complex splitting. Nuclear Magnetic Resonance Use: To assist in the elucidation of a molecule’s structure Information Gained: • Different chemical environments of nuclei being analyzed (1H nuclei): chemical shift • The number of nuclei with different chemical environments: number of signals in spectrum • The numbers of protons with the same chemical environment: integration • Determine how many protons are bonded to the same carbon: integration • Determine the number of protons that are adjacent to one another: splitting patterns • Determine which protons are adjacent to one another: coupling constants Integration • Area underneath signal; NMR machine will give integrals • First, gives the relative ratio of different types of protons in compound • Second, allows determination of actual ratio of different types of protons 1. Measure the length of the integral with a ruler 2. Establish a relative ratio of protons (divide each length by the lowest number) Coupling Constants (J) Protons that split each other’s peaks will have the same coupling constant or J value. Problem: LG 15.2 Draw the structure of a compound that fits each molecular formula and has a 1H NMR spectrum showing a single peak (a singlet). (Hint: Consider HDI). Single peak must mean equivalent Hs! (Only one peak and no splitting.) (a) C2H6O (c) C4H6 (f) C3H6O (g) C4H9Br Problem: LG 21.29 (a-d) Steps to solve problem: 1. Calculate HDI 2. Draw out possibilities of structure 3. Determine how many signals would be seen for each possibility & their splitting patterns 4. Work with the NMR data: (a) Count # of signals = the # of different types of H’s (b) Look at the splitting of each signal (c) Consider the ppm values for each signal - use correlation chart (d) Look at the integration values LG 21.29(a) C4H10O 1.28 ppm (s, 9H) 4.5 ppm (s, 1H) LG 21.29(b) C3H7Br 1.71 ppm (d, 6H) 4.32 ppm (m, 1H) LG 21.29(c) C4H9Cl 1.04 ppm (d, 6H) 1.95 ppm (m, 1H) 3.35 ppm (d, 2H) LG 21.29(d) C8H10 1.25 ppm (t, 3H) 2.68 ppm (q, 2H) 7.23 ppm (m, 5H) LG 21.31 The 1H NMR spectrum of a compound C3H3Cl5 shows peaks at 4.5 ppm (t, 1H) and 6.0 ppm (d, 2H). What is the compound’s structure? IR Correlation Chart Correlation of Bond Stretching and IR Absorption (See also Correlation Chart & Table in Lab Guide) Wavenumber Range (cm-1) Type of Bond Group Family of Compounds Single Bonds —C—H Alkanes 2850-3300 =C—H Alkenes, aromatics 3000-3100 ºC—H Alkynes 3300-3320 O—H Alcohols 3200-3600 N—H Amines 3300-3500 C—O Ethers, Esters, Alcohols Carboxylic Acids 1330-1000 C=C Alkenes, aromatics 1600-1680 C=O Carbonyls 1680-1750 Aldehydes, ketones 1710-1750 Carboxylic acids 1700-1725 Esters, amides 1680-1750 C=N Imines 1500-1650 CºC Alkynes 2100-2200 CºN Nitriles 2200-2300 Double Bonds Triple Bonds 1H NMR Correlation Chart Antioxidants & Chocolate Antioxidants: Protect against cardiovascular disease, cancer and cataracts Thought to slow the effects of aging Chocolate: High levels of antioxidants - complex mixtures of phenolic compounds By weight, has higher concentration of antioxidants than red wine or green tea 20x higher concentration of antioxidants than tomatoes Dark chocolate has more than 2x the level of antioxidants as milk chocolate. Side note: The main fatty acid in chocolate, stearic acid, does not appear to raise blood cholesterol levels the way other saturated fatty acids do.