Chem 2600 Spring 2006 Prof. Peter W. Dibble Lab: Greg Patenaude
advertisement

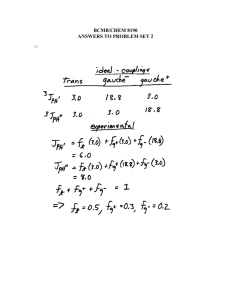
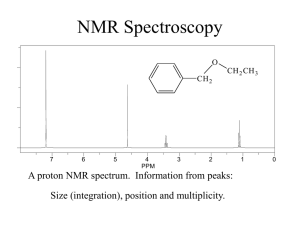
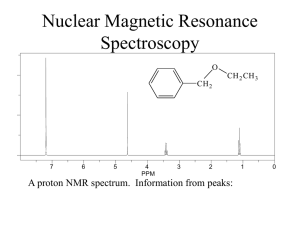
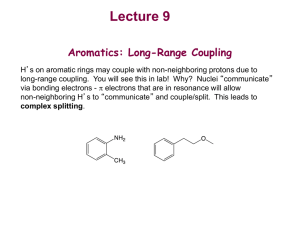
Chem 2600 Spring 2006 Prof. Peter W. Dibble Lab: Greg Patenaude Lab E-810 Ext. 2308 Office: E-858 Ext. 2305; NMR D771B Ext. 2314; Email: Dibble@uleth.ca Class we b site (for downloading class materials): classes.uleth.ca/200601/chem2600a/ or follow the links at classes.uleth.ca Prerequisite: Chem 2500 or permission of instructor. If yo u do no t have the prerequisite yo u will be deregistered. Text: "Organic Chemistry" 2nd. edition, by Dr. Thomas N. Sorrell. In addition to the text yo u will find it very useful to obtain a molecular model kit. You will be allowed to use these kits during all exams in this and (my) future courses. These kits are also useful for Chem 2810. Darling model kits (so named after their creator, not because they are cute) are available from the PCB Club (the Physics, Chemistry and Biology un dergrad uat e society), E-859. The boo kstore sells a different kind of kit for ca. $50.00. Course Outline: (in approx. order, time permitting) NMR Subs titution Reac tions of Alcohols Elimination Reac tions Addition reactions of Alkenes and Alkyne s Addition reactions of conjugated dienes The Chemistry of Benzene Addition Reac tions of Aldehyd es and Ketones Reac tions of Carboxylic acid deriva tives Organometallic chemistry Free Radicals Elementary Mass spectroscopy Special Topics (Drug development) Chapter in Te xt 13 7 8 9 10 12 14 Ev aluation: % Assignments, 4@2.5 10 2 Midterms @ 10 20 (7-9) pm Tuesdays, Feb. 8th and March 8 th L1060) Lab 30 (50% constitutes a pass) Final Exam 40 (Tentatively, Thur sday, April 19th, 9-12am) You will be permitted the use of model kits on your exams. The exercizes and assignments are meant to be partial preparation for the midterms. Ignore them at your great peril. I will be releasing my exam bank wi th answ ers as pdf files. In addition, I will try to post or emai thos e text questions that I feel are representative of wh at yo u sho uld kno w. NOTE: Failure to sit for an exam will result in a mark of zero unless a valid reason for absence is presented. Notify me as soon as possible if yo u are going to miss an exam. If you miss a midterm for a valid reason, that portion of yo ur grade will be added to the final. Students must pass bot h the lab and lecture portions of the course (i.e. a good lab mark will not pull a failing lecture mark up to a pass). IF YOU ARE CAUGHT CHEATING ON ANY EXAM YOU WILL BE ASSIGNED A GRADE OF F FOR THE COURSE INSTANTLY AND A LETTER DESCRIBING YOUR OFFENSE WILL BE PLACED IN YOUR STUDENT FILE. TWO SUCH LETTERS IS GROUNDS FOR EXPULSION FROM THE UNIVERSITY. Check out the calendar, page 63. Nuclear Magnetic Resonance Spectroscopy O CH 2 7 6 5 4 PPM 3 2 CH 2 CH 3 1 A proton NMR spectrum. Information from peaks: magnitude (integration), position and multiplicity. 0 Signal… Lines… Multiplicity… How does NMR it work? Nuclei behave as if they spin… Nuclear spin is quantized and described by the quantum no. I, where I = 0, 1/2, 1, 3/2, 2, …. Spin 1/2 nuclei: 1H (proton), 13C, 19F, 31P Spin 0 nuclei: 12C, 16O Spin 1 nuclei: 14N, 2H Spin 5/2: 17O Spin 3: 10B In a given sample of a compound in solution, spins are random and their fields… In the presence of an externally applied magnetic field (Ho), a nucleus will adopt 2I + 1 orientations with differing energies. For the proton, there are two “spin states”. Ho +1 / 2 -1 / 2 Zeeman splitting -1 / 2 ² E = h E Ho +1 / 2 Important: The relative number of nuclei in the different spin states (proton, 60 MHz) is: E N upper N lower e kT 1,000,000 1,000,009 h E h Ho 2 Ho 2 The magnetogyric ratio, , is a physical constant for each nucleus. 1H 2H 13C 19F 31P So ∆E depends on… Resonance occurs when… 267.53 radians/Tesla* 41.1 67.28 251.7 108.3 * 1 Tesla = 10,000 Gauss. For the proton nucleus: • Ho 14.10 kG 58.74 kG 117,500 kG 60 MHz 250 MHz 500 MHz But… the actual resonance frequency for a given nucleus depends on its… LOCAL CHEMICAL ENVIRONMENT i.e. magnetic interactions within the molecule. These local magnetic effects are due to: • electrons • electrons • other nuclei - especially protons electrons H In the locale of the proton, the field lines are opposed to the applied magnetic field. This has the effect of “shielding” the proton from the full Ho. C He e Ho Chemical shielding has the effect of moving signals to the right on an NMR spectrum. We refer to this as… Deshielding - downfield 7 6 5 4 PPM Shielding - upfield 3 Chemical Shift 2 1 0 The magnitude of He is proportional to the electron density in the bond. He Consider… Ho CH 3 CH 3 H3 C O CH 3 H3 C C CH 3 CH 3 H3 C Si CH 3 CH 3 Tetramethylsilane aka TMS Because almost all proton resonance frequencies are found downfield of the TMS signal, TMS is used as an internal reference. All peak positions are measured as frequencies in Hz downfield of TMS, with TMS at zero. There is a problem however.. is proportional to Ho Ho = 60 MHz, = 162 Hz downfield of TMS Ho = 100 MHz, = 270 Hz downfield of TMS Solution to this problem… We define the d scale: d signal downfi eld of TMS spectrometer frequency in MHz ppm 162Hz 270Hz d 2.70ppm 60MHz 100MHz Chemical shift correlates well with electronegativity… H3 C X F EN 4.0 dppm O 3.5 CHCl 3 CH 2 Cl 2 CH 3 Cl 7 .2 7 5 .3 0 3 .0 5 CH 2 Br 3 .3 0 Cl 3.1 CH2 CH 2 Br 1 .6 9 Br 2.8 I 2.5 H 2.1 CH2 CH 2 CH 2 Br 1 .2 5 TMS 1.8 CH2 CH 2 CH 3 1 .2 0 Electron Effects - Diamagnetic Anisotropy Alkenes… 5.70 e H H H3 C H H C H C H 1.65 5.59 1.65 5.59 5.03 1.71 H 1.96 4.97 1.96 1.90 1.90 4.00 4.60 6.40 O O 6.05 Ho Vinyl protons are downfield, 4.5 - 6 ppm. H OCH 3 5.80 H H 6.43 3.76 Aldehydes 7.45 O H3 C e C 2.20 O 7.54 H 9.72 7.45 R C O H Alkynes H e 1.80 H3 C C C 7.81 C H 1.82 C 7.81 H 9.87 7.26 e 7.26 7.26 7.26 7.26 7.26 H3 C H 2.35 7.06 7.06 7.14 7.14 7.07 X-ray Structure of the 3C Naphthodifuran Cyclophane Chemical shift equivalence … of atoms or groups. Groups or nuclei are shift equivalent if they can be exchanged by a bond rotation without changing the structure of the molecule. H C H H H3 C C CH 3 CH 3 These atoms/groups are said to be “homotopic”. Homotopic atoms/groups are always shift equivalent. Shift equivalence can also be determined by symmetry properties of the molecule. A proper axis of rotation… H3 C C CH 3 CH 3 H H C H CH 3 CH 3 H H Atoms/groups that can be reflected in an internal mirror plane of symmetry, but not exchanged by bond rotation or a proper axis of symmetry are “enantiotopic”. O O C C H H H H H H H H3 C H2 C Cl H H H H C C H H Cl For our current purposes, enantiotopic protons are always shift equivalent. It is easy to recognize atoms/groups that are constitutionally different. O C CH 3 H H H H H H C C H2 CH 2 C HO C H H H Protons that are not constitutionally different, that cannot be exchanged by bond rotation or by molecular symmetry are “diastereotopic”. CH 3 Cl H H H H H3 C H H H H C H H CH 3 H Cl C Cl Cl H Cl Diastereotopic atoms/groups are NOT shift equivalent, except by coincidence (i.e. they happen to have the same chemical shift by accident). O CH 2 CH 3 CH 2 7 6 5 4 PPM Raw integrals: 8.5:3.5:3.4:5.0 3 2 1 0 Other nuclei - spin-spin coupling Consider the vicinal protons in the molecule: HA C Cl Cl HM C Br In the absence of any influence by HM, HA will resonate at A. Br But proton HM generates its own magnetic field which will affect A. Is this field aligned with Ho or against Ho i.e. will it shield or deshield HA? Remember that the population of the two spin states are nearly equal. In the total of all molecules, half of the HM protons will be aligned with Ho and half against. So in half the molecules, HM shields HA and in half HM deshields HA. We therefore see two peaks for HA, of equal intensity; one vA upfield of A and one downfield of A. We call this type of signal a … “doublet”. We say that HA is “split” into a doublet by HM. This effect is referred to as … “spin-spin” coupling. The distance between the two peaks in Hz is J, the coupling constant. J AM Important aspects of coupling: • coupling always goes both ways. If HA is split by HM, then HM must also be split by HA and J must be equal in both cases. • coupling is a through-bond and not a through-space effect. • coupling between shift-equivalent nuclei is not observed. A few words about J. The magnitude of J can be extremely useful in determining structure. It depends on: • number of bonds between nuclei • type of bonds between nuclei • type of nuclei • conformation Because coupling is a through bond effect, the magnitude of J depends on the number of bonds between coupling nuclei. One bond couplings are larger than two bond couplings, two larger than three. In proton-proton couplings, four bond couplings are not usually observed. Geminal H Vicinal J3 = 7 Hz* H H Cl H H Cl H Cl Long range J4 = 0 Hz J2 = 12-14 Hz H H Cl H Cl H * In conformationally averaged systems. Cl Type of bonds… -bonds transmit coupling effects more effectively than bonds. H1 H1 H1 H2 H2 H2 H3 H3 H3 H4 H4 H4 H H H CH 3 The magnitude of vicinal couplings depends strongly on the overlap between adjacent C-H bonds. C H HH H H C H Cl H Cl H H H H Cl H H Cl C C H H H H Cl H H C H H C H H H H H Cl H H H H Consider the following molecule: HA HX C Cl Cl C HY Cl The CH2 has one neighbouring proton in equal proportions of the up and down spin states. This resonance will therefore appear as a … And HA… Possible spin combinations: HX HY So HA will appear as a three line pattern: a triplet, with peak intensities in a 1:2:1 ratio where the lines are separated by J Hz. The central line will occur at the resonance frequency of HA. NOTE: The SIGNAL INTEGRATION tells you about the number of protons that give rise to a particular signal. MULTIPLICITY tells you about the number of … NEIGHBOURING NUCLEI. The n + 1 rule: for simple aliphatic systems, the number of lines in a given signal is n+1 where n is the no. of neighbouring protons. O C H 3 CH 2 C Cl In this molecule, the CH3 protons will appear as a … The CH2 protons have three neighbours. The spin combinations are… Giving rise to a quartet with peak intensities of 1:3:3:1. The combination of a 2 proton quartet and a three proton triplet is characteristic of the presence of an ethyl group. 2 1 PPM 0 The isopropyl group. H H3 C 3 Cl C CH 3 2 PPM 1 0 The n-propyl group. 3 CH 3 CH 2 CH 2 Cl 2 PPM 1 0 The t-butyl group. H2 C Cl C H3 C 3 2 PPM CH 3 CH 3 1 0 Chemical exchange processes - protons attached to O and N. (Alcohols, phenols, carboxylic acids, amines - but not amides) Unlike most spectroscopic methods, the acquisition of signal in NMR spectroscopy takes about three seconds (proton). In that time, protons attached to O or N can be transferred from one molecule to another via the autoionization process… H O H3 C H O H3 C H O O H3 C H3 C H H O O H3 C H H H3 C O H3 C H O H3 C O H3 C H H O H3 C O H3 C H H O H3 C H H3 C O H H3 C O H H3 C O H3 C O If the rate of exchange is slow compared to the time scale of the NMR experiment (I.e. three seconds) then the spectrum is that expected of CH3OH. Under these conditions, vicinal OH:CH coupling is observed. This is rarely the case. If the rate of exchange is comparable to the NMR time scale, then one observes the exchanging proton in a range of environments and at a range of chemical shift postions. HHH H HHH H H O CH O 3 H3 C H Under these conditions, the OH peak is broad and coupling is not observed… H The rate of exchange is catalyzed by acids and bases, depends on solvent, temperature, concentration, purity, and lunar phase. Exchangeable protons… • do not couple • have variable shift positions • are observed as broad peaks • exchange with D2O OH Alcohols 1-5 ppm Phenols 3.5-6 ppm Carboxylic acids 10-12 ppm My personal record 13 ppm. NH (amines) 0.5 - 5 ppm 3 2 PPM 1 0 2 1 PPM 0 Occasionally, carboxylic acid protons resonances are so broad … ..that the only way to tell that they are there is to integrate the baseline. D2O exchange… Exchangeable protons on a molecule will exchange with exchangeable protons on other molecules… D2 O O H3 C H This can be very useful. HOD O H3 C D Coupling in non n+1 systems. Substituted benzenes. 1,4 X These always appear as perfectly symmetrical patterns that look like two doublets or a quartet. CN Br Y Symmetrical 1,2 disubstituted benzenes These always appear as a perfectly symmetrical pattern with a lot of fine structure … X Cl Cl X 1,2,4- trisubstituted systems and the dreaded tree diagrams. CN H6 H2 H5 Cl OH J5,6 = 6 Hz J2,6 = 2 Hz J2,5 = 0 Hz 6 2 5 Coupling in non n+1 systems. Monosubstituted benzenes. X If the substituent is an alkyl group or halogen, then all five protons tend to show up in the same place as either a singlet or a somewhat broad singlet. If X is a strong electron-withdrawing group (carbonyl, nitro, sulfonic acid) then the ortho and para protons will be pulled downfield. X If X is a strong electron-donating group (OH, OR, NH2) then the ortho and para protons are pushed upfield. OH