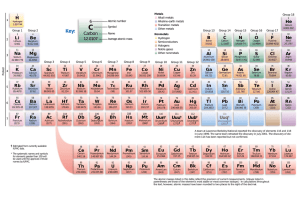
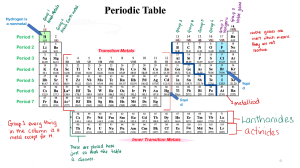
Definition - Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalized. Characteristics –One Capital letter or one Capital letter followed by one lower case letter. Atomic Symbol Examples – Mg Ne O Non- examples – H2O Definition – Characteristics - Atomic Number Examples - Non Examples - Definition – Characteristics - Atomic Mass Examples - Non Examples - Definition – Characteristics - Groups Examples - Non Examples - Definition – Characteristics - Period Examples - Non Examples - Definition – Characteristics - Reactivity Examples - Non Examples - Definition – Characteristics - Valence Electrons Examples - Non Examples - Definition – Characteristics - Metals Examples - Non Examples - Definition – Characteristics - Non Metals Examples - Non Examples - Definition – Characteristics - Metaliods Examples - Non Examples - Definition – Characteristics - Alkali Metals Examples - Non Examples - Definition – Characteristics - Alkali Earth Metals Examples - Non Examples - Definition – Characteristics - Transition Metals Examples - Non Examples - Definition – Characteristics - Halogens Examples - Non Examples - Definition – any of the gaseous elements helium, neon, argon, krypton, xenon, and radon, occupying Group 0 (18) of the periodic table. They were long believed to be totally unreactive but compounds of xenon, krypton, and radon are now known. Characteristics - ; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. Noble Gases Examples – He-Helium , Ar – Argon, Ne –Neon Non Examples – K – Potasium, Fe - Iron