Phase Changes
advertisement

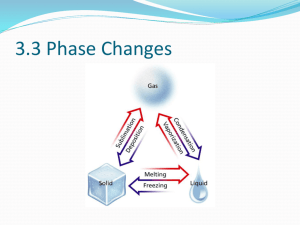
States of Matter Section 12.4: Phase Changes Objectives ► Explain how the addition and removal of energy can cause a phase change. ► Interpret a phase diagram. Phase Changes ► Most substances can exist in 3 states depending on the temperature and pressure. ► Water exists in 3 states on Earth - ice (solid), water (liquid), and water vapor (gas). ► States are referred to as phases when they coexist; for example, ice water has 2 phases, ice and liquid water. Phase Changes & Energy •When energy is added or removed from a system, one phase can change into another. •Melting, vaporization & sublimation require energy. •In deposition, condensation, & freezing, energy must be removed. Melting (or Fusion) ► Heat (the transfer of energy from an object at a higher temperature to an object at a lower one) moves from the water into the ice. In this case, when it is assumed the temperature of the ice and water are the same at 0 0C, the energy absorbed by the ice is NOT used to raise its temperature. Melting (or Fusion) ► The energy absorbed by the ice goes into breaking the hydrogen bonds between the water molecules in the solid. The molecules can then move apart and become liquid. Melting (or Fusion) ► Water has many hydrogen bonds to break, so a lot of energy is needed to melt it (change the solid to liquid). Different substances will require different energies. Ionic compounds (with ionic bonds) will require more energy. ► The melting point of a crystalline solid is the temperature at which the forces holding the crystal lattice together are broken and it becomes a liquid. ► Every element and compound has a different melting point. The Heating Curve for Water ► As the ice (in our ice water) melts, ITS TEMPERATURE REMAINS CONSTANT. ► Once it is all melted, additional energy increases the kinetic energy of the molecules and the water temperature increases. ►Molecules with enough energy can escape the liquid and become a gas. ►Vaporization is the process by which a liquid changes to a gas or vapor. Vaporization ► As more energy is added, more molecules escape to the gas phase. As this occurs, THE TEMPERATURE DOES NOT CHANGE. ► When all molecules are in the gas phase, additional energy will cause an increase in temperature. What is evaporation? ► When vaporization takes place only at the surface of the of the liquid, the process is called evaporation. ► This can happen at LOW temperatures, especially if there is light energy. VAPOR PRESSURE ►The pressure exerted by a vapor over a liquid is called vapor pressure. ►An increase in temperature will increase the vapor pressure. Vapor Pressure Curves ► ► A vapor pressure curve shows how the vapor pressure of a liquid (yaxis) changes as a function of temperature (x-axis). When the curve intersects the point where the vapor pressure equals the atmospheric pressure (760 mm Hg), the temperature is called the normal boiling point. What is boiling? ► The temperature at which the vapor pressure of a liquid equals the external or atmospheric pressure is called the BOILING POINT (or normal boiling point). What is boiling? ► At the boiling point, molecules throughout the liquid have enough energy to vaporize. Bubbles of vapor can be seen in the liquid. ► Bubbles then rise to the surface . Sublimation ► Sublimation is when a solid substance changes directly to a gas. ► Solid iodine, carbon dioxide (dry ice), mothballs, and solid air fresheners sublime at room temperature. Ice cubes sublime in your freezer. Condensation ► When water vapor molecules lose energy (perhaps by contact with a cold object), their velocity decreases. They are then more likely to form hydrogen bonds when they collide with other water molecules, thus forming a liquid. ► The process by which a gas or a vapor becomes a liquid is called condensation. Deposition ► Deposition is the process by which a substance changes from a gas or vapor to a solid without first becoming a liquid. ► Energy is released to the surroundings as ice crystals form from water vapor. Freezing (or Solidification or Crystallization) ► ► ► As heat flows from water to its surroundings, the water molecules lose kinetic energy. Formation of hydrogen bonds will take place which will keep the molecules fixed into set positions. A solid will form. The freezing point is the temperature at which a liquid is converted into a crystalline solid. . A New Look at the Heating Curve ► Read this curve from RIGHT TO LEFT - take away heat instead of adding it. ► Condensation occurs at the same temperature as vaporization. ► Freezing occurs at the same temperature as melting. Phase Diagrams ►2 variables control the phase of a substance. Temperature Pressure ►A phase diagram is a graph of pressure vs. temperature. It will show which phase a substance will be in at various conditions of temperature & pressure. Phase Diagram Different sections of the graph indicate different phases. Phase Diagram ► Boundaries between the sections are the temperature & pressure conditions at which a phase change is occurring. ► The triple point is the point on a phase diagram that represents the temperature and pressure at which 3 phases of a substance can coexist. Phase Diagram ► The critical point indicates the critical pressure and critical temperature above which water cannot exist as a liquid. ► At the critical temperature, an increase in pressure will not change a vapor into a liquid. Phase Diagram ► The phase diagram for each substance is different because the normal boiling and freezing points of substances are different. Water Carbon Dioxide