Cells - Seattle Central College
advertisement

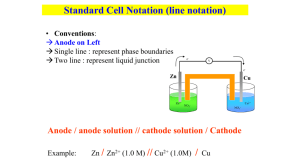
Chapter 19 Electrochemistry Electrochemistry Electrochemistry is the branch of chemistry that examines the transformations between chemical and electrical energy. Electricity is the movement of electrons from one point to another. The chemical change responsible for producing electrons is called oxidation. Since matter cannot be created nor destroyed then the electrons must go somewhere, thus causing a reduction. Electrochemistry Electrochemistry is the application of spontaneous and nonspontaneous reactions in our wonderful world. Spontaneous reactions most often are found in batteries, called galvanic cells while nonspontaneous reactions are used to plate metals on top of metals called electrolytic cells. Oxidation-Reduction Reactions Oxidation Zn(s) ---> Zn2+(aq) + 2e- Reduction Cu2+(aq) + 2e- --> Cu(s) Oxidizing Agent, the substance reduced Reducing Agent, the substance oxidized Voltaic Cell (Galvanic Cell) A spontaneous REDOX reaction where the electrons are forced to get to the substance reduced by flowing through an external circuit. Cells An electrochemical cell is an apparatus that converts chemical energy into electrical work or electrical work into chemical energy. In a voltaic cell, chemical energy is transformed into electrical energy by a spontaneous redox reaction. This is a battery. A cell diagram uses symbols to show how the components of an electrochemical cell are connected. Parts of Cells • An anode is an electrode at which an oxidation half-reaction (loss of electrons) takes place. • A cathode is an electrode at which a reduction half-reaction (gain of electrons) takes place. • A salt bridge connects the two solutions of the cell to prevent a short-circuit. Standard Potentials • Standard Potentials (Eo) is the electromotive force of a half-reaction in which all reactants and products are their standard states. • Standard Cell Potentials (Eocell) is the electromotive force produced by an electrochemical cell when all reactants and products are in their standard states. • Standard States - concentrations are 1M and the partial pressure of gases are 1 bar. • Eocell = Eocathode + Eoanode Determining Standard Potentials Standard reduction potentials are measured relative to a hydrogen electrode with defined voltage of 0.00 volts The hydrogen electrode Hydrogen gas is bubbled in at one atmosphere over a platinum electrode Since ions cannot serve as an electrode then platinum is used (inert) Connected via a salt bridge and wire with a volt meter is another electrode Determining Standard Potentials Typically any metal, with the it’s metal ion in solution to the extent of 1.0M The volt meter measures the potential difference between the two cells and is recorded as the standard voltage The voltage of the hydrogen cell is defined to be 0.0 at standard conditions Standard conditions Room temperature 25⁰C 1.0 M ion concentration 1.0 atm pressure Standard Hydrogen Electrode SHE is defined as 0.000V Determination of Eo with SHE 0.76 V = ESHE - EZn 0.76 V = 0.00 V - EZn 0.34 V = ECu - ESHE 0.34 V = Ecu - 0.00 V Writing a Cell Diagram 1. Write the chemical symbol of the anode at the far left and the symbol of the cathode at the far right. 2. Work from the electrodes toward the bridge using vertical lines to indicate phase changes and the symbols of ions or compounds that are changed by the cell reaction. 3. Use a double vertical line to represent the bridge connecting the anode and cathode half-reactions. Cell Diagram Example 1. Zn(s) . . . . . . Cu(s) 2. Zn(s) | Zn2+(aq). . .Cu2+(aq) | Cu(s) 3. Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s) Practice Write the voltaic-cell reaction and sketch a cell in which magnesium metal is oxidized to magnesium ions and copper ions are reduced to copper metal. Identify the cathode, anode, and direction of the electron flow on the sketch. Practice Ag+ + e- Ag Cu - 2e- Cu2+ - 0.34v 0.80v Practice 2(Ag+ + eCu - 2e2 Ag+ + Cu Ag) 0.80v Cu2+ - 0.34v Ag + Cu2+ Practice 2(Ag+ + eCu - 2e2 Ag+ + Cu Ag) 0.80v Cu2+ - 0.34v Ag + Cu2+ V = 0.46 volts Practice 2(Ag+ + eCu - 2e2 Ag+ + Cu Ag) 0.80v Cu2+ - 0.34v Ag + Cu2+ V = 0.46 volts Where is the anode? Practice anode 2(Ag+ + eCu - 2e2 Ag+ + Cu Ag) 0.80v Cu2+ - 0.34v Ag + Cu2+ V = 0.46 volts Where is the anode? Where is the cathode? Practice cathode anode 2(Ag+ + eCu - 2e2 Ag+ + Cu Ag) 0.80v Cu2+ - 0.34v Ag + Cu2+ V = 0.46 volts Where is the anode? Where is the cathode? What is the purpose of the salt bridge? cathode anode Practice 2(Ag+ + eCu - 2e2 Ag+ + Cu Ag) 0.80v Cu2+ - 0.34v Ag + Cu2+ V = 0.46 volts Where is the anode? Where is the cathode? What is the purpose of the salt bridge? To keep the cells neutral Cell Thermodynamics Cell potential, Electrical Work, and Free Energy In order to push electrons through a wire a force must be present to push the electrons. It is called the electromotive force EMF and is measured in volts. A volt is j/C, one joule of force is required to push one mole of electrons between two points of a circuit having different potential energies. When the system does work on the surroundings it is considered to be negative, while work done on the system is positive. Cell Thermodynamics Note that cell potential and work have opposite signs V=-w/q, where q is the charge in coulombs Work here is negative, since we are referring to the system. Since ΔE = q + w and if there is no loss of heat then V is maximum work possible. Since entropy of the universe is expanding, then maximum work cannot occur, since heat is released to expand the universe. Maximum potential can be measured, without current flowing, with a potentiometer or an efficient digital voltmeter. No current flow implies no wasted energy Cell Thermodynamics The maximum potential difference is useful to compare process in order to determine their efficiency. Faraday is the charge of one mole of electrons which is 96485 C/mole e Sample Problem Calculate the work to push 5.97 moles of electrons with a voltage of 2.10 volts. 2.10j -W = C 96485C 5.97 mole e kj = 1210 Kj mole e 103j kj 96485C 5.97 mole e 2.50j -Wmax = C mole e 103 j = 1440 Kj Cell Thermodynamics The maximum potential difference is useful to compare process in order to determine their efficiency. Faraday is the charge of one mole of electrons which is 96485 C/mole e Sample Problem % efficiency = 1210 X100 = 84.0% 1440 Calculate the work to push 5.97 moles of electrons with a voltage of 2.10 volts. 2.10j -W = C 96485C 5.97 mole e kj = 1210 Kj mole e 103j kj 96485C 5.97 mole e 2.50j -Wmax = C mole e 103 j = 1440 Kj Voltage and Free Energy G = -nFEcell Cell Potential (Ecell) or Electromotive Force is the voltage between the electrodes of a voltaic cell (#V = #J/C; C - coulomb). Faraday constant (F) is 9.65 x 104 C/(mol e-) n - number of moles of electrons Voltage and Electrical Work Gcell = wmax = -qEmax = -nFEmax welec = Gcell = -nFEcell ∆G=∆G°+RTlnQ Voltage and Electrical Work Gcell = wmax = -qEmax = -nFEmax welec = Gcell = -nFEcell ∆G=∆G°+RTlnQ -nFE = -nFE° + RTlnQ Voltage and Electrical Work Gcell = wmax = -qEmax = -nFEmax welec = Gcell = -nFEcell ∆G=∆G°+RTlnQ E = E° +RT/nF [lnQ] -nFE = -nFE° + RTlnQ E = E° - 0.0591 logQ n Cell Potential and Concentration Consider the reaction: Al(s) + 3 Mn3+ → 2 Al3+ + 3 Mn(s) E° = 0.48 v What would happen if the concentration of Mn3+ were increased greater than 1.0 M? The reaction would shift right Shifting right makes a smaller ∆G Should produce more volts What would happen if the concentration of Mn3+ were decreased less than 1.0 M? Concentration Cells What would happen if two silver half cells had different concentrations of silver ions? Electrons would spontaneous flow in a direction so as to make the concentrations equal to each other V=0 or ∆G= 0 Electrons should flow from the less concentrated to the more concentrated half cell Examples of concentration cells • • • • In animals Potato batteries Different areas of the ocean Voltage and Electrical Work Gcell = wmax = -qEmax = -nFEmax welec = Gcell = -nFEcell ∆G=∆G°+RTlnQ -nFE = -nFE° + RTlnQ E = E° +RT/nF [lnQ] E = E° - 0. 591 logQ n Nernst Equation Remember K=Q at equilibrium Note: RTlnQ = 0.0591logQ Concentration Cells Calculate the initial voltage of the following cell: Zn(s) | 0.1M Zn2+(aq) || 0.2 Zn2+(aq) | Zn(s) 0.0591 2+ log Zn 2 1 0.0591 log Ecathode = E° 2 Zn2+ Eanode = E° - Concentration Cells Calculate the initial voltage of the following cell: Zn(s) | 0.1M Zn2+(aq) || 0.2 Zn2+(aq) | Zn(s) 0.0591 2+ log Zn 2 1 0.0591 log Ecathode = E° 2 Zn2+ Eanode = E° - Subtract bottom equation from top equation 2+ Zn 0.0591 log Zn2+ Eanode – Ecathode = - 2 Concentration Cells Calculate the initial voltage of the following cell: Zn(s) | 0.1M Zn2+(aq) || 0.2 Zn2+(aq) | Zn(s) 0.0591 2+ log Zn 2 1 0.0591 log Ecathode = E° 2 Zn2+ Eanode = E° - Subtract bottom equation from top equation 0.1 0.0591 log Eanode – Ecathode = 0.2 2 Concentration Cells Calculate the initial voltage of the following cell: Zn(s) | 0.1M Zn2+(aq) || 0.2 Zn2+(aq) | Zn(s) 0.0591 2+ log Zn 2 1 0.0591 log Ecathode = E° 2 Zn2+ Eanode = E° - Subtract bottom equation from top equation 0.1 0.0591 log = 0.9 V Eanode – Ecathode = 0.2 2 Ion-Selective Electrodes Because cell potential is sensitive to the concentrations of the reactants and products involved in the cell reaction, measured potentials can be used to determine concentration of an ion. pH meters are an example of this. A glass electrode contains a reference cell, plus a thin glass membrane at the end that measure ion concentrations. The membrane can be made sensitive to ions other than H, just by varying the type of glass. pH Meter Pt | H2(g, 1atm)| H+(xM) || H+ (1M) | H2 (g 1atm) | Pt pH Meter Eanode – Ecathode = - 0.0591 2 Eanode – Ecathode = - 0.0591 2 [H+]2 log 2 1 2 log [H+] 12 [H+] Eanode – Ecathode = - 0.0591 log 12 + Eanode – Ecathode = - 0.0591 log [H ] Ecell = 0.0591 pH Ecell pH = 0.0591 Calculating Equilibrium constants • • • • • ∆G=∆G°+RTlnQ -nFE°=-RTlnEeq ∆G°=-RTlnKeq RTlnKeq E°= nF Use the measured voltage for E Use the E° voltage Calculate K The Dry Cell Anode: Zn (s) → Zn2+ (aq) + 2 eCathaode: 2MnO2(s) + 2 H+ → Mn2O3 + HOH 2MnO2(s) + 2 NH4+ + → Mn2O3 +2 NH3+ HOH Cell Reaction: Zn (s) + 2MnO2(s) + 2 NH4+ → Zn + Mn2O3 + 2NH3 + HOH Lead Storage Battery Pb2+ PbO2 Pb Pb - 2ePbO2 + 4H+ + 2 ePb + PbO2 + 4H+ Pb2+ Pb2+ + 2H2O 2Pb2+ + 2H2O (Anode Rx) (Cathode Rx) (Cell Rx) Lead Storage Battery Charging Electron flow A Pb2+ Pb PbO2 charging Pb - 2ePb2+ PbO2 + 4H+ + 2 ePb2+ + 2H2O Pb + PbO2 + 4H+ 2 Pb2+ + 2H2O (Anode Rx) (Cathode Rx) (Cell Rx) Fuel Cell Cathode: Anode: H2 → 2H+ + 2 e- ½ O2 + 2H+ +2e- → HOH A cell that converts chemical energy directly into electrical energy Similar to a battery, except the reactants are continually supplied from an external source. PEM Fuel Cell The anode is separated from the cathode by a proton exchange membrane (PEM) Only protons can pass through, while the electrons are forced through an external circuit. On the other side of the PEM protons, electrons, and oxygen combine to form water Only about 0.7 volts per cell, thus many cells are linked in series. Much current research into PEM membranes. Corrosion: Unwanted Voltaic Cells Corrosion can be viewed as returning metals to their natural stable state Corrosion involves oxidation of a metal Since one fifth of the steel produced annually replaces rusted metal Metals oxidize easily, since their outer shell electrons are loosely held Oxidation of metals by oxygen is a spontaneous process giving a positive voltage, or a negative free energy; gold and platinum are the exceptions. Corrosion: Unwanted Voltaic Cells Most metals form a thin oxide coating protecting the inner metal atoms from oxidation, such as aluminum, magnesium, nickel, and chromium Copper will produce an exterior green carbonate (called patina), while silver produces Ag2S, where does the sulfur come from? Corrosion: Unwanted Voltaic Cells Consider a water droplet on the surface of steel. Some of the iron dissolves into the water to produce Fe2+ ions The lost electrons flow along the steel where they come into contact with moist oxygen to form hydroxide ions. Corrosion: Unwanted Voltaic Cells Finally the Fe2+ reaches some oxygen, being further oxidized and converted into Fe2O3 Moisture must be present to help transport ions much like a salt bridge. Corrosion: Unwanted Voltaic Cells The iron oxide then combines with water to make various hydrates, thus different colors of rust Corrosion Prevention Prevention of corrosion is an important way of conserving our natural resources of energy and metals. • Primary means is paint • Chromium and tin are often used to plate steel because they oxidize fo form a durable, effective oxide coating • Zinc, also used to coat steel a process called galvanizing (mixed oxide carbonate coating) Since zinc is a more active metal, losses it electrons more easily, then any oxidation dissolves zinc rather than iron. • Corrosion Prevention • Alloying is also used to prevent corrosion Stainless steel contains chromium and nickel, both of which form oxide coatings that change steel’s reduction potential to one characteristic of the noble metals Ion bombardment is being used to produce a thin layer of stainless steel on the surface, thus cheaper Cathodic Protection When another metal more easily oxidized is electrically connected to the metal that is being protected Cathodic Protection For example the Alaska pipeline is protected by wire wrapped around the pipe and connected to magnesium pounded into the ground Cathodic Protection Reduction (water) happens at the cathode and is called cathodic protection; making steel act as a cathode and therefore does not oxidize Alaska Pipeline O2 + 2H2O + 4e → 4OHEº=0.40 2(Mg - 2e → Mg 2+) Eº= 2.38 v O2 + 2 H2O + 2 Mg Mg2+ + 4 OH- Ecell = 2.88 v Electrolytic Cells A nonspontaneous reaction When REDOX reactions are forced to go in the opposite direction Electrolysis is used to plate metals, or to purify metals, or produce chemicals Salt bridge is not necessary, but a battery is required Platinum electrodes are used Voltage must add to a negative value Electrolytic Cells Electrollysis of salt water Possible Anode Reactions H2O - 4eO2 + 4H+ v = -1.23 2Cl - - 2e- Cl2 v = -1.36 Possible Cathode Reactions HOH + 2eNa+ + e- H2 + OH - v= -0.83 Na+ v=-2.71 Electrolytic Cells Electrollysis of salt water Possible Anode Reactions H2O - 4eO2 + 4H+ v = -1.23 2Cl - - 2e- Cl2 v = -1.36 Possible Cathode Reactions HOH + 2eNa+ + e- H2 + OH - v= -0.83 Na+ v=-2.71 Which combinations do we choose? Electrolytic Cells Electrolysis of salt water Possible Anode Reactions H2O - 4eO2 + 4H+ v = -1.23 2Cl - - 2e- Cl2 v = -1.36 Possible Cathode Reactions HOH + 2eNa+ + e- H2 + OH - v= -0.83 Na+ v=-2.71 Which combinations do we choose? One of each having the smallest voltage, except overvoltage (0.4-0.6v) must be supplied to over come high energy of activation forming gas on a metal surface. In light of this, chlorine is oxidized and water is reduced to produce chlorine gas at the anode and hydrogen gas at the cathode. Electrolytic Cells Electrolysis of salt water 2Cl - - 2e- HOH + 2e2Cl - + HOH Cl2 v = -1.36 (anode Rx_ H2 + OH- v= -0.83 (cathode Rx) H2 + Cl2 V=-2.19 (cell Rx) Electrolytic Cells Electrolysis of salt water 2Cl - - 2e- HOH + 2e2Cl - + HOH Cl2 v = -1.36 (anode Rx_ H2 + OH- v= -0.83 (cathode Rx) H2 + Cl2 + OH- V=-2.19 (cell Rx) Note: Use inert electrodes (Pt) and a battery larger than 2.19 volts. What do you think might happen if phenolphthalein were added to the electrolyte? Yes, a pink color would develop around the cathode and eventually spread through the solution Electrolytic Cells Electrolysis of salt water 2Cl - - 2e- HOH + 2e2Cl - + HOH Cl2 v = -1.36 (anode Rx_ H2 + OH- v= -0.83 (cathode Rx) H2 + Cl2 + OH- V=-2.19 (cell Rx) Note: Use inert electrodes (Pt) and a battery larger than 2.19 volts. What do you think might happen if phenolphthalein were added to the electrolyte? Electroplating How long must a current of 5.00 A be applied to a solution of Ag+ to produce 12.0 g of silver on the spoon in the figure? Amp=C/s Ag+ + e- Ag (s) Electroplating How long must a current of 5.00 A be applied to a solution of Ag+ to produce 12.0 g of silver on the spoon in the figure? Amp=C/s Ag+ + e- Ag (s) Electroplating How long must a current of 5.00 A be applied to a solution of Ag+ to produce 12.0 g of silver on the spoon in the figure? Amp=C/s Ag+ + e- amp-s C Ag (s) Electroplating How long must a current of 5.00 A be applied to a solution of Ag+ to produce 12.0 g of silver on the spoon in the figure? Amp=C/s Ag+ + e- Ag (s) amp-s C 5.00 amp Electroplating How long must a current of 5.00 A be applied to a solution of Ag+ to produce 12.0 g of silver on the spoon in the figure? Amp=C/s Ag+ + e- amp-s C Ag (s) 96500C 5.00 amp mole e- Electroplating How long must a current of 5.00 A be applied to a solution of Ag+ to produce 12.0 g of silver on the spoon in the figure? Amp=C/s Ag+ + e- amp-s C Ag (s) 96500C mole e5.00 amp mole e- mole Ag Electroplating How long must a current of 5.00 A be applied to a solution of Ag+ to produce 12.0 g of silver on the spoon in the figure? Amp=C/s Ag+ + e- amp-s C Ag (s) 96500C mole e5.00 amp mole e- mole Ag Electroplating How long must a current of 5.00 A be applied to a solution of Ag+ to produce 12.0 g of silver on the spoon in the figure? Amp=C/s Ag+ + e- amp-s C Ag (s) 96500C mole e5.00 amp mole e- mole Ag mole Ag 107.9 g Ag Electroplating How long must a current of 5.00 A be applied to a solution of Ag+ to produce 12.0 g of silver on the spoon in the figure? Amp=C/s Ag+ + e- amp-s C Ag (s) 96500C mole e5.00 amp mole e- mole Ag mole Ag 12.0 g Ag 107.9 g Ag Electroplating How long must a current of 5.00 A be applied to a solution of Ag+ to produce 12.0 g of silver on the spoon in the figure? Amp=C/s Ag+ + e- amp-s C Ag (s) 96500C mole e5.00 amp mole e- mole Ag mole Ag 12.0 g Ag hr 107.9 g Ag 3600 s Electroplating How long must a current of 5.00 A be applied to a solution of Ag+ to produce 12.0 g of silver on the spoon in the figure? Amp=C/s Ag+ + e- amp-s C Ag (s) 96500C mole e5.00 amp mole e- mole Ag mole Ag 12.0 g Ag hr 107.9 g Ag 3600 s = 0.596 hrs Commercial Applications Charles Martin Hall (1863-1914) was a student at Oberlin College in Ohio when he first became interested in aluminum. One of his professors commented that anyone who could manufacture aluminum cheaply would make a fortune. Aluminum Production Aluminum Production Aluminum is the third most abundant element on the earth Since aluminum is a very active metal, it is found in nature as its oxide in and ore called bauxite Production of aluminum metal from its ore proved to be more difficult than of most other metals Reduction of ions requires dissolving the ions so that they can migrate to electrodes Water is not suitable, since it is more easily reduced compared to aluminum Aluminum Production Cryolite (Na3AlF6) was the solvent that finally worked to dissolve the aluminum oxide Aluminum oxide melts at 2000°C, but a mixture of cryolite and aluminum oxide melts at 1000°C The anode, made of graphite, oxidizes the oxide to oxygen gas The cathode the inner shell of the cell also made of graphite reduces the aluminum to molten metal, which is drained off from the bottom Aluminum Production Thus aluminum can be reduced electrolytically A mixture of aluminum oxide and crolite melts at about 1000°C Bauxite is reacted with OH- to remove other metal oxides since they will not dissolve Production of Copper An interesting application is the refining, or purification of copper metal Impure copper is used as the anode in an electrolytic cell containing aqueous copper sulfate as the electrolyte. The cathode of the cell is made of pure copper. When electrolysis is carried out the voltage across the cell is adjusted so that only copper and other more active metals, such as iron or zinc are able to dissolve at the anode. Production of Copper Since copper is the ion most easily reduced, then it is the only ion plated on the pure copper electrode The other ions stay in solution The sludge that does not dissolve, called anode sludge, contains gold, platinum and silver Down’s cell Down’s cell Made of brick, since high temperature to melt NaCl The anode pokes up from the floor, with a hood over it to capture the chlorine gas The cathode, doughnut shaped, around the anode, with a pipe in the top to drain off the sodium metal, which is less dense than the molten sodium chloride ChemTour: Zinc-Copper Cell Click to launch animation PC | Mac This ChemTour illustrates the reactions that occur at the electrodes of a typical zinc–copper battery and explores how the energy released by a voltaic cell is used to do work on the surroundings. ChemTour: Free Energy Click to launch animation PC | Mac Students learn how the potential of an electrochemical cell can be used to determine the free energy available to do work, and explore the relationships between free energy, cell potential, and the equilibrium constant. ChemTour: Cell Potential Click to launch animation PC | Mac This ChemTour explores the concept of cell potential (Ecell) as a measure of how much electrical energy is stored in an electrochemical cell. ChemTour: Alkaline Battery Click to launch animation PC | Mac This ChemTour explores the oxidation–reduction reactions that power common alkaline batteries and describes the changes in reaction quotient as a battery loses its charge. ChemTour: Fuel Cell Click to launch animation PC | Mac Students learn how fuel cells use a redox reaction between hydrogen and oxygen to produce electrical energy. Given that the standard reduction potential of Ag+ (aq) is 0.80 V, will a silver rod dissolve in an acidic solution of pH 0.0 under standard conditions? A) Yes Immersion of Ag Rod in Acid Solution B) No C) Depends Given that the standard reduction potential of Ag+ (aq) is 0.80 V, will a silver rod dissolve in an acidic solution of pH 0.0 under standard conditions? Ag+ + e- A) Yes Immersion of Ag Rod in Acid Solution Ag (s) B) No 0.80 V C) Depends Given that the standard reduction potential of Ag+ (aq) is 0.80 V, will a silver rod dissolve in an acidic solution of pH 0.0 under standard conditions? Ag+ + e- Ag (s) 0.80 V Oxidation or reduction? A) Yes Immersion of Ag Rod in Acid Solution B) No C) Depends Given that the standard reduction potential of Ag+ (aq) is 0.80 V, will a silver rod dissolve in an acidic solution of pH 0.0 under standard conditions? Ag+ + e- Ag (s) 0.80 V Oxidation or reduction? Reduction! Dissolving? A) Yes Immersion of Ag Rod in Acid Solution B) No C) Depends Given that the standard reduction potential of Ag+ (aq) is 0.80 V, will a silver rod dissolve in an acidic solution of pH 0.0 under standard conditions? Ag+ + e- Ag (s) 0.80 V Oxidation or reduction? Reduction! Dissolving? No, Dissolving is oxidation A) Yes Immersion of Ag Rod in Acid Solution B) No C) Depends Given that the standard reduction potential of Ag+ (aq) is 0.80 V, will a silver rod dissolve in an acidic solution of pH 0.0 under standard conditions? Ag (s) + e- Ag+ (aq) - 0.80 V Dissolving is oxidation and is non spontaneous. A) Yes Immersion of Ag Rod in Acid Solution B) No C) Depends Given that the standard reduction potential of Ag+ (aq) is 0.80 V, will a silver rod dissolve in an acidic solution of pH 0.0 under standard conditions? Ag (s) + e- Ag+ (aq) - 0.80 V Dissolving is oxidation and is non spontaneous. A) Yes Immersion of Ag Rod in Acid Solution B) No C) Depends Under standard conditions, copper will plate out onto a nickel rod immersed in a solution containing Cu2+ ions, but aluminum will not plate onto a nickel rod immersed in a solution containing Al3+ ions. Which of the following is the strongest oxidizing agent? A) Ni(s) B) Al3+(aq) Oxidizing Strength of Al3+, Ni, and Cu2+ C) Cu2+(aq) Under standard conditions, copper will plate out onto a nickel rod immersed in a solution containing Cu2+ ions, but aluminum will not plate onto a nickel rod immersed in a solution containing Al3+ ions. Which of the following is the strongest oxidizing agent? Cu2+ + Ni Ni2+ + Cu E°>0 Ni2+ + Al E°<0 o.a. Al3+ + Ni o.a A) Ni(s) B) Al3+(aq) Oxidizing Strength of Al3+, Ni, and Cu2+ C) Cu2+(aq) Under standard conditions, copper will plate out onto a nickel rod immersed in a solution containing Cu2+ ions, but aluminum will not plate onto a nickel rod immersed in a solution containing Al3+ ions. Which of the following is the strongest oxidizing agent? Cu2+ + Ni Ni2+ + Cu E°>0 Ni2+ + Al E°<0 o.a. Al3+ + Ni o.a A) Ni(s) B) Al3+(aq) Oxidizing Strength of Al3+, Ni, and Cu2+ C) Cu2+(aq) Under standard conditions, copper will plate out onto a nickel rod immersed in a solution containing Cu2+ ions, but aluminum will not plate onto a nickel rod immersed in a solution containing Al3+ ions. Which of the following is the strongest oxidizing agent? Cu2+ + Ni Ni2+ + Cu E°>0 Ni2+ + Al E°<0 o.a. Al3+ + Ni o.a A) Ni(s) B) Al3+(aq) Can Ni(s) ever be a reducing agent? Oxidizing Strength of Al3+, Ni, and Cu2+ C) Cu2+(aq) Under standard conditions, copper will plate out onto a nickel rod immersed in a solution containing Cu2+ ions, but aluminum will not plate onto a nickel rod immersed in a solution containing Al3+ ions. Which of the following is the strongest oxidizing agent? Cu2+ + Ni Ni2+ + Cu E°>0 Ni2+ + Al E°<0 o.a. Al3+ + Ni o.a A) Ni(s) B) Al3+(aq) C) Cu2+(aq) Can Ni(s) ever be a reducing agent? Only if it can reduce. Ever hear of Ni2-? Oxidizing Strength of Al3+, Ni, and Cu2+ For the reaction Cu2+(aq) + 2 Ag(s) Cu(s) + 2 Ag+(aq), G° = 88.3 kJ/mole. Under standard conditions, will the Cu/Ag cell pictured to the left produce a current? A) Yes Ag/Cu Electrochemical Cell B) No C) Depends For the reaction Cu2+(aq) + 2 Ag(s) Cu(s) + 2 Ag+(aq), G° = 88.3 kJ/mole. Under standard conditions, will the Cu/Ag cell pictured to the left produce a current? Is the forward reaction spontaneous? A) Yes Ag/Cu Electrochemical Cell B) No C) Depends For the reaction Cu2+(aq) + 2 Ag(s) Cu(s) + 2 Ag+(aq), G° = 88.3 kJ/mole. Under standard conditions, will the Cu/Ag cell pictured to the left produce a current? Is the forward reaction spontaneous? No! A) Yes Ag/Cu Electrochemical Cell B) No C) Depends For the reaction Cu2+(aq) + 2 Ag(s) Cu(s) + 2 Ag+(aq), G° = 88.3 kJ/mole. Under standard conditions, will the Cu/Ag cell pictured to the left produce a current? How about the reverse reaction? A) Yes Ag/Cu Electrochemical Cell B) No C) Depends For the reaction Cu2+(aq) + 2 Ag(s) Cu(s) + 2 Ag+(aq), G° = 88.3 kJ/mole. Under standard conditions, will the Cu/Ag cell pictured to the left produce a current? How about the reverse reaction? Yes A) Yes Ag/Cu Electrochemical Cell B) No C) Depends For the reaction Cu2+(aq) + 2 Ag(s) Cu(s) + 2 Ag+(aq), G° = 88.3 kJ/mole. Under standard conditions, will the Cu/Ag cell pictured to the left produce a current? How about the reverse reaction? Yes Then copper will oxidize producing e’s A) Yes Ag/Cu Electrochemical Cell B) No C) Depends For the reaction Cu2+(aq) + 2 Ag(s) Cu(s) + 2 Ag+(aq), G° = 88.3 kJ/mole. Under standard conditions, will the Cu/Ag cell pictured to the left produce a current? How about the reverse reaction? Yes Then copper will oxidize producing e’s A) Yes Ag/Cu Electrochemical Cell B) No C) Depends For the oxidation of Fe2+ by Ag+, Fe2+(aq) + Ag+(aq) Fe3+(aq) + Ag(s), H° and S° are both negative. Which of the following plots shows the correct relationship between the electromotive force, E°, (y-axis) and the temperature (xaxis)? A) Oxidation of Fe + by Ag+ B) C) For the oxidation of Fe2+ by Ag+, Fe2+(aq) + Ag+(aq) Fe3+(aq) + Ag(s), H° and S° are both negative. Which of the following plots shows the correct relationship between the electromotive force, E°, (y-axis) and the temperature (xaxis)? ΔG = ΔH - TΔS A) Oxidation of Fe + by Ag+ B) C) For the oxidation of Fe2+ by Ag+, Fe2+(aq) + Ag+(aq) Fe3+(aq) + Ag(s), H° and S° are both negative. Which of the following plots shows the correct relationship between the electromotive force, E°, (y-axis) and the temperature (xaxis)? ΔG = ΔH - TΔS Spontaneous or nonspontaneous? A) Oxidation of Fe + by Ag+ B) C) For the oxidation of Fe2+ by Ag+, Fe2+(aq) + Ag+(aq) Fe3+(aq) + Ag(s), H° and S° are both negative. Which of the following plots shows the correct relationship between the electromotive force, E°, (y-axis) and the temperature (xaxis)? ΔG = ΔH - TΔS Yes spontaneous at low T A) Oxidation of Fe + by Ag+ B) C) For the oxidation of Fe2+ by Ag+, Fe2+(aq) + Ag+(aq) Fe3+(aq) + Ag(s), H° and S° are both negative. Which of the following plots shows the correct relationship between the electromotive force, E°, (y-axis) and the temperature (xaxis)? ΔG = ΔH - TΔS Yes spontaneous at low T A) Oxidation of Fe + by Ag+ B) C) For the cell pictured to the left, the voltage, E, is measured to be 0.80 V. Given the following standard reduction potentials, Ag+ + e→ Ag 2 H+ + 2 e- → H2 E red= 0.80 V E red = 0.00 V what can be said of the pH in the right half of the cell if the partial pressure of H2(g) is 1.0 atm? A) pH > 0 pH of an Electrochemical Cell B) pH = 0 C) pH < 0 For the cell pictured to the left, the voltage, E, is measured to be 0.80 V. Given the following standard reduction potentials, Ag+ + e→ Ag 2 H+ + 2 e- → H2 E red= 0.80 V E red = 0.00 V what can be said of the pH in the right half of the cell if the partial pressure of H2(g) is 1.0 atm? Oxidation occurs in which half cell? A) pH > 0 pH of an Electrochemical Cell B) pH = 0 C) pH < 0 For the cell pictured to the left, the voltage, E, is measured to be 0.80 V. Given the following standard reduction potentials, Ag+ + e→ Ag 2 H+ + 2 e- → H2 E red= 0.80 V E red = 0.00 V what can be said of the pH in the right half of the cell if the partial pressure of H2(g) is 1.0 atm? Oxidation occurs in which half cell? Yes, the hydrogen cell A) pH > 0 pH of an Electrochemical Cell B) pH = 0 C) pH < 0 For the cell pictured to the left, the voltage, E, is measured to be 0.80 V. Given the following standard reduction potentials, Ag+ + e→ Ag 2 H+ + 2 e- → H2 E red= 0.80 V E red = 0.00 V what can be said of the pH in the right half of the cell if the partial pressure of H2(g) is 1.0 atm? In order to have 0.00 V in the hydrogen half cell, what must the proton concentration be? A) pH > 0 pH of an Electrochemical Cell B) pH = 0 C) pH < 0 For the cell pictured to the left, the voltage, E, is measured to be 0.80 V. Given the following standard reduction potentials, Ag+ + e→ Ag 2 H+ + 2 e- → H2 E red= 0.80 V E red = 0.00 V what can be said of the pH in the right half of the cell if the partial pressure of H2(g) is 1.0 atm? In order to have 0.00 V in the hydrogen half cell, what must the proton concentration be? Yes, one molar A) pH > 0 pH of an Electrochemical Cell B) pH = 0 C) pH < 0 For the cell pictured to the left, the voltage, E, is measured to be 0.80 V. Given the following standard reduction potentials, Ag+ + e→ Ag 2 H+ + 2 e- → H2 E red= 0.80 V E red = 0.00 V what can be said of the pH in the right half of the cell if the partial pressure of H2(g) is 1.0 atm? In order to have 0.00 V in the hydrogen half cell, what must the proton concentration be? Yes, one molar. And the pH? A) pH > 0 pH of an Electrochemical Cell B) pH = 0 C) pH < 0 For the cell pictured to the left, the voltage, E, is measured to be 0.80 V. Given the following standard reduction potentials, Ag+ + e→ Ag 2 H+ + 2 e- → H2 E red= 0.80 V E red = 0.00 V what can be said of the pH in the right half of the cell if the partial pressure of H2(g) is 1.0 atm? In order to have 0.00 V in the hydrogen half cell, what must the proton concentration be? Yes, one molar. And the pH? A) pH > 0 pH of an Electrochemical Cell B) pH = 0 C) pH < 0 For the electrochemical cell Cu | Cu2+(1.0 M) || Cu2+(0.1 M) | Cu, what will happen to the color in the darker solution (1.0 M Cu2+) once the circuit is completed? This is a concentration cell and electrons flow from one half cell to the other until the solutions have equal concentrations. A) It gets darker. B) It gets lighter. C) It stays the same. Cu/Cu + Concentration Cell For the electrochemical cell Cu | Cu2+(1.0 M) || Cu2+(0.1 M) | Cu, what will happen to the color in the darker solution (1.0 M Cu2+) once the circuit is completed? This is a concentration cell and electrons flow from one half cell to the other until the solutions have equal concentrations. A) It gets darker. B) It gets lighter. C) It stays the same. Cu/Cu + Concentration Cell The End