Electrochem III
advertisement

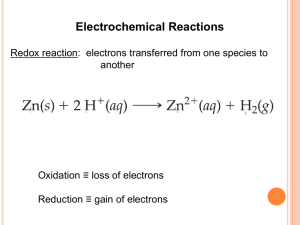
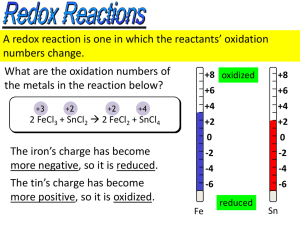
Standard Reduction Potentials Electrochem III Balancing Oxidation-Reduction Equations Perhaps the easiest way to balance the equation of an oxidationreduction reaction is via the half-reaction method. Balancing Oxidation-Reduction Equations This involves treating the oxidation and reduction as two separate processes, balancing these half reactions, and then combining them to attain the balanced equation for the overall reaction. The Half-Reaction Method 1.Assign oxidation numbers to determine what is oxidized and what is reduced. 2.Write the oxidation and reduction halfreactions. The Half-Reaction Method 3.Balance each halfreaction. • • • • Balance elements other than H and O. Balance O by adding H2O. Balance H by adding H+. Balance charge by adding electrons. 4.Multiply the half- reactions by integers so that the electrons gained and lost are the same. The Half-Reaction Method 5.Add the half- reactions, subtracting things that appear on both sides. Make sure the equation is balanced according to mass. Make sure the equation is balanced according to charge. 6. 7. The Half-Reaction Method Consider the reaction between MnO4− and C2O42− : MnO4− (aq) + C2O42− (aq) Mn2+ (aq) + CO2 (aq) The Half-Reaction Method • First, we assign oxidation numbers. +7 +3 +2 +4 MnO4− + C2O42- Mn2+ + CO2 • Manganese is oxidized. • Carbon is reduced. The Half-Reaction Method C2O42− CO2 To balance the carbon, we add a coefficient of 2: C2O42− 2 CO2 The Half-Reaction Method C2O42− 2 CO2 The oxygen is now balanced as well. To balance the charge, we must add 2 electrons to the right side. C2O42− 2 CO2 + 2 e− The Half-Reaction Method MnO4− Mn2+ The manganese is balanced; to balance the oxygen, we must add 4 waters to the right side. MnO4− Mn2+ + 4 H2O The Half-Reaction Method MnO4− Mn2+ + 4 H2O To balance the hydrogen, we add 8 H+ to the left side. 8 H+ + MnO4− Mn2+ + 4 H2O The Half-Reaction Method 8 H+ + MnO4− Mn2+ + 4 H2O To balance the charge, we add 5 e− to the left side. 5 e− + 8 H+ + MnO4− Mn2+ + 4 H2O The Half-Reaction Method Now we evaluate the two half-reactions together: C2O42− 2 CO2 + 2 e− 5 e− + 8 H+ + MnO4− Mn2+ + 4 H2O To attain the same number of electrons on each side, we will multiply the first reaction by 5 and the second by 2. The Half-Reaction Method 5 C2O42− 10 CO2 + 10 e− 10 e− + 16 H+ + 2 MnO4− 2 Mn2+ + 8 H2O When we add these together, we get: 10 e− + 16 H+ + 2 MnO4− + 5 C2O42− 2 Mn2+ + 8 H2O + 10 CO2 +10 e− The Half-Reaction Method 10 e− + 16 H+ + 2 MnO4− + 5 C2O42− 2 Mn2+ + 8 H2O + 10 CO2 +10 e− The only thing that appears on both sides are the electrons. Subtracting them, we are left with: 16 H+ + 2 MnO4− + 5 C2O42− 2 Mn2++ 8 H2O + 10 CO2 Half Reactions in Basic Solutions If a reaction occurs in basic solution, one can balance it as if it occurred in acid. Once the equation is balanced, add OH− to each side to “neutralize” the H+ in the equation and create water in its place. If this produces water on both sides, you might have to subtract water from each side. • • • • • Standard Cell Potentials A galvanic cell is always a redox reaction that has been separated into 2 half reactions. Each half reaction is assigned a potential from which the cell potential can be derived. • For example: • Redox rxn: 2H (aq) + Zn(s) Zn • The Cell Potential is 0.76 V + Anode Zn (s) Zn2+ (aq) +2e- 2+ (aq) + H2 (g) Cathode 2H+(aq) + 2e- H2 (g) Standard Hydrogen Electrode Definition: When the platinum cathode is this half reaction has a concentration of 1 M [H+] with H2 gas at 1 atm. We can measure Ecell of the total cell at 0.76 V, but there is no way to measure each half reaction directly. To deal with this, the standard hydrogen electrode has been assigned a cell potential of 0.0V. Since the Standard Hydrogen Electrode is assigned 0.0V and the total Ecell = 0.76V, then Zn(s) Zn2+(aq) +2e- has a potential of 0.76 V Why??? Because E°cell= E°cathode +E°Anode E°cell= E°H+H2+ E°Zn Zn2+ The ° indicates standard states are employed with 2H+(aq) + 2e- H2 (g) as 0.0V We can assign values to all other half reactions. For example: Zn (s) + Cu 2+ Zn 2+ + Cu (s) What are the half reactions? Anode Zn(s) Zn2+ (aq) + 2eCathode Cu (aq) + 2e- Cu (s) LEO GER Zn (s) + Cu 2+ Zn 2+ + Cu (s) E°cell= E°cathode +E°Anode The E°cell is measured at 1.10 V, And E°Anode is determined that Zn(s) Zn2+ (aq) + 2e- is 0.76V, Then E°cathode Cu (aq) + 2e- Cu (s) must be 0.34 V Because 1.10V = 0.76 V + 0.34 V measured known calculated The value of the standard hydrogen electrode. The value of the standard hydrogen electrode of 0.0V is accepted by the scientific community. Standard Reduction Potentials When the cell potentials of half reactions are given, they are written as: Standard Reduction Potentials: All half reactions have solutions at 1 M and gases at 1 atm. Zn2+ (aq) + 2e- Zn (s) Cu+ (aq) + e- Cu (s) 2H+(aq) + 2e- H2 (g) They are all written as a gain of electrons, reduction. GER Combining 2 half reactions to obtain a balanced oxidation-reduction reaction require 2 manipulations: 1.One of the reactions must be reversed, which means the sign of its E° must be reversed. 2.Since the number of electrons lost by the anode must equal the number of electrons gained by the cathode. The half reactions are multiplied to find the lowest common value. But the value of the E° does not change!! Note: Standard reduction potentials are intensive properties. It does not matter how many times the reaction occurs, only that it occurs. Consider a Galvanic Cells Based on the unbalanced Reaction Fe 3+ (aq) + Cu (s) Cu 2+ (aq) + Fe 2+ (aq) The two half reactions are as standard reduction potentials: Fe 3+ (aq) + 1e- Fe 2+ (aq) E° = 0.77V Cu 2+ (aq) + 2e- Cu (s) E° = 0.34V E°cell continued To balance the cell reaction and calculate the E° equation number 2 must be reversed and the sign of the E° must be reversed. Cu (s) Cu 2+ (aq) + 2eE°= -0.34V And one must double the first equation to balance the electrons. 2Fe 3+(aq) + 2e- 2Fe 2+(aq) E°=0.77V NOTE: The value of E°is not multiplied Sum up the two half reactions Cu (s) Cu 2+ (aq) + 2eE°= -0.34V 2Fe 3+(aq) + 2e- 2Fe 2+(aq) E°=0.77V 2Fe3+(aq)+Cu(s) 2Fe2+(aq)+Cu2+(aq) E°=0.43V Other Examples www.animationfactory.com Backdrops: - These are full sized backdrops, just double click them and size it up! Images: - Most of these .gifs, .jpgs, and .png images can be scaled up to fit your needs! Title Backdrop Slide Backdrop Print Backdrop Transitional Backdrop