Organic - Course
advertisement

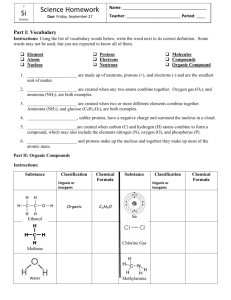
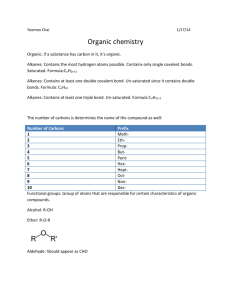
Gabriela Lopez Organic Chemistry ( Oh! Chem.) Organic – contains carbon, # of organic comp > # of inorganic compounds b/c carbon atom has 4 potential sites for covalent bonding. - Single bonded organic compound are said to be saturated. - Double and Triple covalent bond are unsaturated. Molecular Formula (ex: C3H8) Structural Formula (ex: H = C =H ) - Organic comps classified into groups called Homologous series Hydrocarbons only contain hydrogen atoms. Three types Alkanes, Alkenes, and Alkynes Alkanes: single bond, saturated hydrocarbons that release energy when burned. - Increase # of carbons in alkane series increase boiling pt - Increment Formula: CnH2n+2 Alkenes: double bond, unsaturated hydrocarbon Increment Formula: CnH2n Alkynes: triple bond, unsaturated hydrocarbons Increment Formula: CnH2n-2 -------------------------------------------------------------------------------------------------------------------Isomers: compounds with different molecular formula but the same structural arrangement. Different chemical and physical properties. As # of carbons increase # of possible isomers increases Straight –chain hydrocarbons are called normal form. Ex: (n-butane) Naming Alkanes with branches: 1. Find parent alkane (the longest continuous chain) 2. Find the branched (alkyl group) and name it corresponding to its alkane 3. State location of branch: Parent carbon chain must be numbered from the end that will give the branch its lowest #. 4. Use a prefix if there is more than one of the same type of branched group attached to the parent chain. ________________________________________________________________________ Functional Groups: when other atoms replace hydrogen in a hydrocarbon Halides: any halogen (F, Cl, Br, or I) replaces a hydrogen. Also known as halocarbon. - Named by citing the location of halogen in the attached chain. Alcohols: compounds in which one or more hydrogens are replaced by an –OH group. (hydroxyl group). –OH does not form an ion in water but are very polar! Polarity allows alcohol to be soluble in water. - Type of alcohol depends on the # of –OH groups in the compound and position of –OH group on parent chain. *Classification of Alcohols by POSITION on chain: classified as primary, secondary, and tertiary. # Refers to the number of carbons DIRECTLY attached to the carbon atom with the –OH group. Ex: primary means only one carbon atom is attached to the carbon with the –OH group. Secondary means only two carbons are attached to the carbon with the –OH group. Tertiary means that only three carbon atoms are attached to the carbon with the –OH group. *Dihydroxy and Trihydroxy Alcohols (# of –OH groups in main chain): Common name: Ethylene glycol -> commercial name: Anit-freeze -> Real name: 1,2-ethanediol -> Type of alcohol: dihydroxy Other Substituted Hydrocarbons: Aldehydes: Oxygen double bonded to the end of parent carbon chain (1st or last carbon atom). This part of the aldehyde compound is known as carbonyl group. Naming: 1.) substitute the –e ending of corresponding alkane to –al . Ketones: ~ Aldehydes but the Oxygen double is not at the end of main chain. Naming: 1.) substitute the –e ending of corresponding alkane to –one. 2.) state location of oxygen on chain by using a #. - Ketones: used as solvents, VERY polar -> dissolve in water. Ethers: two carbon chains joined by an oxygen atom. Naming: 1.) name the two carbon chains and replace their endings to –yl. 2.) Put the word ether at the end of the whole name. Organic acids: functional group -> COOH , where the carbon atom at the end chain is double bonded to Oxygen and has another separate single bond to an –OH group. Generally weak electrolytes. Naming: 1.) substitute the –e ending of corresponding alkane to –oic acid. Esters: Formula: R-CO-O-R’ , the R-CO-O part comes from an organic acid. The R’ comes from an alcohol. Have strong fragrant aromas*. Naming (general formula name -> alkyl ,alknoate) : 1.) find parent chain by finding the side with the carbon attached to the Ester functional group. Also change ending to – oate. 2.) Name the rest of the chain and consider it the alkyl group. Amines: formed when one or more of hydrogen atoms of ammonia are replaced by an alkyl group (?). This orangic compound is one of the few that always contain nitrogen. Present in hormones and anesthetics. Naming: 1.) substitute the –e ending of corresponding alkane to –amine. 2.) Name location of alkyl group containing nitrogen by a #. Amino acids: contains an amine group that is adjacent to acid group (10 essential amino acids and building block of protein) Amides: when Acid + AcidWater + Amide (this reaction is also known as Condensation linkage of the 2 acids is Peptide bonding - Amide functional group is located at the end ? of chain. Naming: 1.) Count # of carbons. 2.) Change –e ending to –amide. _________________________________________________________ Organic Reactions: more slowly than inorganic, require catalyst, and must break relatively strong existing bonds before making new bonds. - Combustion: burning of organic comp in presence oxygen water + CO2 + HEAT (complete combustion) OR water + CO (incomplete combustion). Both Exothermic!! - Fermentation: Sugar (from for example fruits) anaerobically digested by yeast (zymase enzyme) and excretes as Ethanol and CO2. The alcohol produce can be used as fuel ex: ethanol. Sugar + (yeast with enzyme) Alcohol + CO2 - Saponification: when ester reacts with an inorganic base to produce an alcohol and soap. Common type of this type of reaction: Fat (animal fat?) + Strong base (ex: NaOH, KOH) Glycerol and Soap - Substitution: replacement of one or more of the hydrogen atoms in a saturated hydrocarbon with another atom or group, most common halogens. C2H6 + Cl2C2H5CI + HCI (or C2H4CI2 + H2) - Addition: Adding one or more atoms at a double or triple bond. You have to break these bonds of this unsaturated comp. In the end you have an alkane. C2H4 + CI2 C2H4CI2 (saturated comp ) - Esterification: rnx btw organic acid and an alcohol to produce an ester and water Organic acid + Alcohol Ester + H2O