Name: _______________________________________ Per:___ Date: ______________
A MODEL ATOM
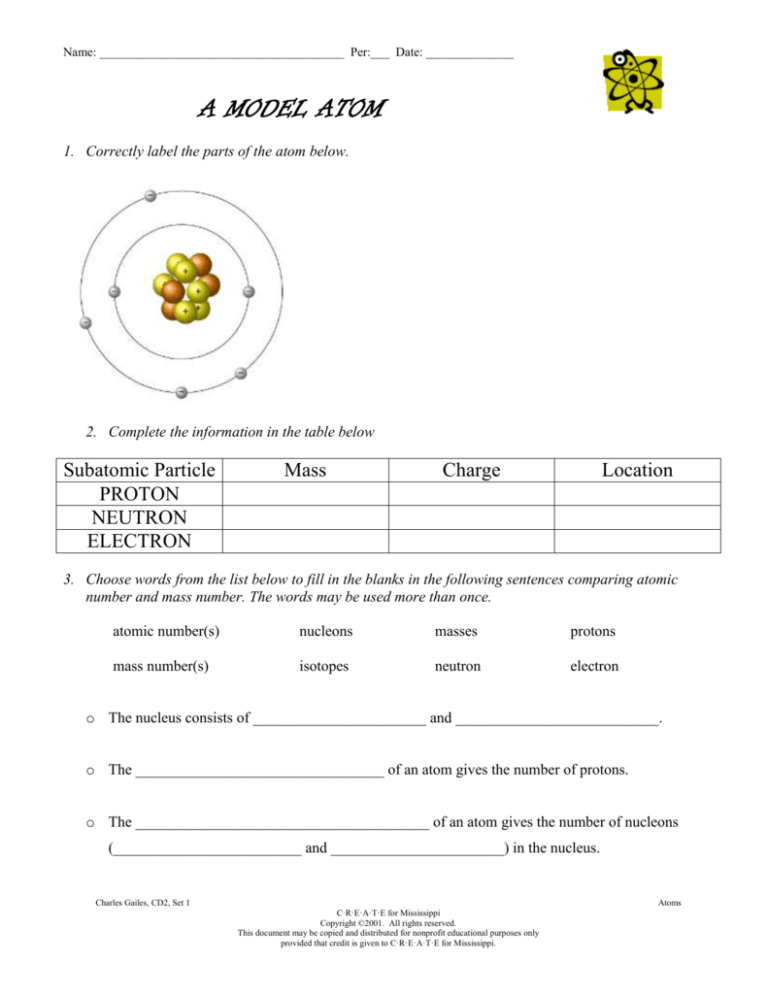
1. Correctly label the parts of the atom below.
2. Complete the information in the table below
Subatomic Particle
PROTON
NEUTRON
ELECTRON
Mass
Charge
Location
3. Choose words from the list below to fill in the blanks in the following sentences comparing atomic
number and mass number. The words may be used more than once.
atomic number(s)
nucleons
masses
protons
mass number(s)
isotopes
neutron
electron
o The nucleus consists of _______________________ and ___________________________.
o The _________________________________ of an atom gives the number of protons.
o The _______________________________________ of an atom gives the number of nucleons
(_________________________ and _______________________) in the nucleus.
Charles Gailes, CD2, Set 1
Atoms
C·R·E·A·T·E for Mississippi
Copyright ©2001. All rights reserved.
This document may be copied and distributed for nonprofit educational purposes only
provided that credit is given to C·R·E·A·T·E for Mississippi.
Name: _______________________________________ Per:___ Date: ______________
o The symbols for the elements are often written with the __________________________________
at the upper left and the ________________________________________ at the lower left.
For example, the symbol
23
Na represents a sodium atom with a _______________________ of
23 and a ___________________________ of 11.
There are 11 ___________________ and 12______________________ in the nucleus of this atom.
Since atoms begin neutral (with no overall charge), the number of positive subatomic particles,
which are called ______________________ is equal to the number of negative subatomic particles,
which are called ______________________, so there are 11 _____________________ as well.
o Atoms of the same element may differ in the number of ___________________ in their nuclei.
o Atoms that have the same ____________________________________ but different numbers of
neutrons are ____________________ of the same element.
3. Fill in the information missing from the following table:
Symbol
Atomic
Number
Mass
Number
Number of
Protons
Numbers of
Electrons
Number of
Neutrons
H
1
1
1
1
0
H
1
3
1
1
2
Na
11
23
B
5
11
1
1
3
1
23
11
11
5
34
S
16
32
S
16
Charles Gailes, CD2, Set 1
Atoms
C·R·E·A·T·E for Mississippi
Copyright ©2001. All rights reserved.
This document may be copied and distributed for nonprofit educational purposes only
provided that credit is given to C·R·E·A·T·E for Mississippi.