Acid/Base Studies - kelliparrinstructionaltech
advertisement

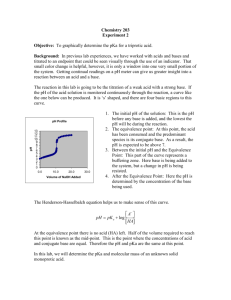
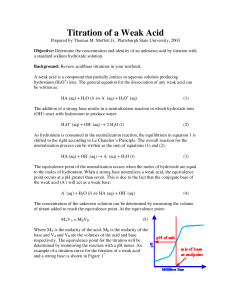
Acid/Base Studies By: Kelli Parr April 12, 2010 General Chemistry 32-106 Introduction: This laboratory experiment focuses on acid/base titrations. A titration involves adding a solution of know concentration to a solution of unknown concentration until the reaction is complete.1 In this lab the solution with a known concentration is 0.10 M NaOH. The solutions of unknown concentration are vinegar, solution A and solution B. The goal of the first part of the lab was to find the molarity of Acetic Acid in vinegar solution through titration. In the later parts of the lab the goal was to find the identities of solutions A and B. The equivalence points of titrations were crucial in solving the goals of the experiment. The equivalence point is when a stoicheometric equivalent amount of base has been added to an acid.2 In this experiment, when halfway to the equivalence point the ratio of base to acid is one to one. Using the Henderson-Hasselbalch equation, pH=pKa+log(base/acid)3, at this halfway point pH will equal pKa. The pKa ia the negative log of the Ka , acid ionization constant. Each acid has its own Ka value that can be used to differentiate it from other acids. Determining the pKa graphically will allow for the identification of the unknown solution. Some acids have one Ka value and two equivalence points, these are called monoprotic acids. These only give a single H+ per unit acid. Others have two Ka values and two equivalence points and give two H+ per unit acid. Experimental Method: First, 10 mL of vinegar was poured into a graduated cylinder. From the graduated cylinder, 5.00 mL was transferred into a 150 mL beaker using a 5.00 mL volumetric pipette. Using a squirt bottle, approximately 15 mL of deionized water was added to the beaker as well. A 50.00 mL burette was filled with 0.10 M standardized NaOH and set over a Fisher Scientific stir and hot plate using burette clamps. The beaker was placed on the stir and hot plate and a stirring bead was placed inside. The plate was set to stir. A Vernier pH sensor was inserted into the beaker so that it would not be hit by the stirring bead and was connected by a clamp. The pH sensor was connected to an iMac computer using a Vernier LabPro interface. The LoggerPro computer program was used to collect the pH data given by the sensor. The 0.10M NaOH was titrated until the pH was about 12. 5mL increments were used at all times during this portion of the experiment except for when the graph showed a steep incline. At this point 1mL was the increment. Between each addition the keep button was pressed on LoggerPro and the next addition wasn’t added until the pH reading was stable. After the first titration was complete, 15mL of solution A was placed into a graduated cylinder. From the graduated cylinder, 10.00mL of solution A was transferred to a 150mL beaker using a 10.00mLvolumetric pipette. Using a squirt bottle about 15mL of deionized water was added to the beaker. The beaker was placed over the stirring plate and the pH sensor was inserted. The 50mL burette was filled to the top line with standardized 0.10 M NaOH. The titration was undergone until the pH reached 12. 1mL increments were used except for the steep portions of the graph were drops were single drops were added. The previous paragraph was then repeated replacing solution A with solution B. Results: Four graphs were made during this experiment. Graphs 1,3 and 4 show the of vinegar, solution A, or solution B with NaOH. Graph 2 shows the equivalence point of the titration of vinegar and NaOH. This was done by converting the y axis from graph 1 to (pH/volume). The point on the graph represented the equivalence point for the solution. Equivalence point graphs were analyzed for graphs 3 and 4 but are not physically included in the report. The overall goal from graphs 1 and 2 were to find the molarity of Acetic Acid in vinegar solution. Graph 1 shows the pH results along with the volume for the titration of vinegar with NaOH. Graph 1: Graph 2, gives a more defined approximation of the equivalence point which was found to be 45.8mL. Graph 2 Knowing the equivalence point and the molarity of the NaOH allowed for the moles of OH- to be calculated. Calculation 1: 0.0456 L × (0.10 moles NaOH/ 1L) = 0.0046 moles OHAt the equivalence point the moles of OH- equals the moles of H+ so the moles of H+ is also 0.0046. Dividing the moles of H+ by the number of liters of Acetic Acid equaled the molarity of Acetic Acid in vinegar solution. Calculation 2: 0.0046 moles H+ / 0.00500 L Acetic Acid = 0.92 M Acetic Acid in vinegar solution The purposes of graphs 3 and 4 were to use the equivalence points to determine the pKa values to then identify solutions A and B. Graph 3 represents the titration of solution A with NaOH. There were two equivalence points for graph 3 so it is a diprotic acid. Graph 3: When the y-axis was changed to show (pH/volume) the equivalence point was found to be 9.94mL. The pH value when the equivalence is divided by 2 equals the pKa value. Calculation 3: 9.94mL /2 = 4.97mL The pH value at 4.97 mL was 2.84. Graph 4 shows the titration of solution B with NaOH. There are two equivalence points for the graph so it is also a diprotic acid. Graph 4: The equivalence point for this solution was found to be 10.14mL. Finding the pH at half of the equivalence point gives the pKa value for solution B. Calculation 4: 10.14mL/2 = 5.07mL The pH and therefore pKa for solution B was 3.32. Discussion: According to calculation 2 the concentration of Acetic Acid in vinegar was found to be 0.92M. The pKa value for solution A was determined to be 2.84. This number closely relates to the pKa value for Malonic acid which is 2.83.4 Graph 3 gave two equivalence points which means it is a diprotic acid. Malonic acid is also diprotic which supports it as the acid for solution A. Graph 4 for solution B was also of a diprotic acid. From this graph the pKa was found to be 3.32. The conclusion for the identity of solution B is Maleic acid. Maleic acid has a pKa value of 1.83. This conclusion was decided because first equivalence point in graph 4 is very faint and hardly distinguishable. An error could have been made while using the pipette to measure and transfer solution B into the beaker. The beaker could have also not been cleaned off well enough and had traces of other solutions in it. Another source for error could have been having too large of increments when adding the NaOH. Finally the mixture could have not been mixed thoroughly enough so an increase in stirring could have resulted in more accurate results. References: 1. Chang, R. General Chemistry: The Essential Concepts, 5th Edition.; McGraw-Hill: Boston 2003, 2.New Mexico State University. Chemistry Department. Titrations. http://www.chemistry.nmsu.edu/studntres/chem116/notes/titrations.html (accessed 4-12-10) 3. University of Calgary. Chemistry/Science: Structure and pKa http://www.chem.ucalgary.ca/courses/351/Carey/Useful/pka.html (accessed 4-12-10) 4. Penn State University. Penn State Department of Chemistry: pKa Data Table Compiled by R. Williams. http://research.chem.psu.edu/brpgroup/pKa_compilation.pdf (accessed 4-12-10)