WORD
advertisement
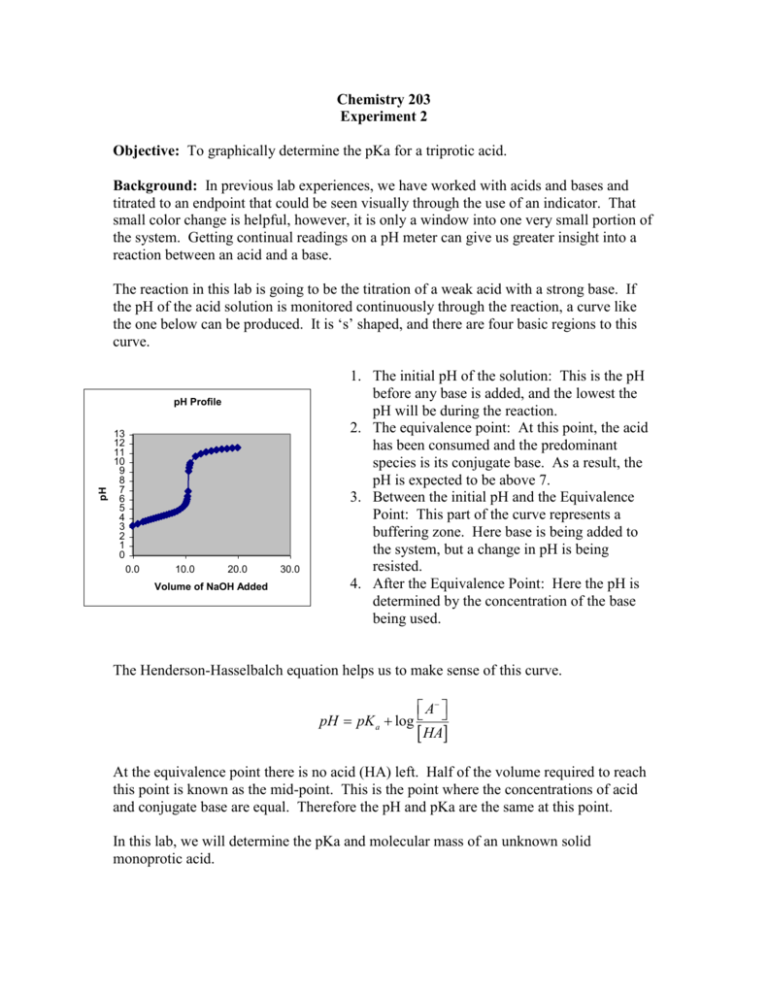
Chemistry 203 Experiment 2 Objective: To graphically determine the pKa for a triprotic acid. Background: In previous lab experiences, we have worked with acids and bases and titrated to an endpoint that could be seen visually through the use of an indicator. That small color change is helpful, however, it is only a window into one very small portion of the system. Getting continual readings on a pH meter can give us greater insight into a reaction between an acid and a base. The reaction in this lab is going to be the titration of a weak acid with a strong base. If the pH of the acid solution is monitored continuously through the reaction, a curve like the one below can be produced. It is ‘s’ shaped, and there are four basic regions to this curve. pH pH Profile 13 12 11 10 9 8 7 6 5 4 3 2 1 0 0.0 10.0 20.0 Volume of NaOH Added 30.0 1. The initial pH of the solution: This is the pH before any base is added, and the lowest the pH will be during the reaction. 2. The equivalence point: At this point, the acid has been consumed and the predominant species is its conjugate base. As a result, the pH is expected to be above 7. 3. Between the initial pH and the Equivalence Point: This part of the curve represents a buffering zone. Here base is being added to the system, but a change in pH is being resisted. 4. After the Equivalence Point: Here the pH is determined by the concentration of the base being used. The Henderson-Hasselbalch equation helps us to make sense of this curve. A pH pK a log HA At the equivalence point there is no acid (HA) left. Half of the volume required to reach this point is known as the mid-point. This is the point where the concentrations of acid and conjugate base are equal. Therefore the pH and pKa are the same at this point. In this lab, we will determine the pKa and molecular mass of an unknown solid monoprotic acid. Procedure: Standardization of Sodium Hydroxide Solution 1. Prepare approximately one liter of 0.02M NaOH using solid NaOH and the top loading balances in the lab. This solution will be standardized and used to titrate the unknown solution later. 2. Clean a buret, and wash at least three times with the sodium hydroxide solution. Fill it to the top mark. 3. Obtain about 450mL of deionized water and boil it for 5 minutes to remove any trace amounts of dissolved carbon dioxide. 4. Place between 0.1g and 0.2g of KHP (potassium hydrogen phthalate) into three Erlenmeyer flasks. This mass should be known 0.0001g. Use the analytical balances and mass by difference. 5. Add about 100mL of the boiled deionized water and 2-3 drops of phenolphthalein indicator to each of the flasks. 6. Titrate the acid with the base until a slight pink color persists. Determine the Ka1 and Ka2 of a triprotic acid (H3PO4) 1. Obtain 10.00mL of 0.025M H3PO4. 2. Pour this into a 150mL beaker and add another 20mL of deionized water. 3. Titrate with the standardized base. Make sure to make smaller additions when the pH goes through large changes. 4. Repeat with another sample. Data The previous procedure asked you to record a number of masses in your notebook. These included: Standardization of NaOH Mass of solid KHP used in each standardization trial. Volume of titrant used. Titration of an unknown acid Volume of base used to titrate. The pH of the solution as base was added. Report From this recorded data, you are required to determine the following: The average concentration of the standardized base. The standard deviation (error) of the standardized base. The pKa’s for Phosphoric Acid. In a 1-2 page report please give the following An abstract describing the reaction, and the experimental pKa’s for phosphoric acid. One titration curve. A brief answer to the questions below. Questions: What would have happened if you titrated phosphoric acid using phenolphthalein as an indicator (pH range 8.3 – 10.0)? Would you have been able to accurately predict the equivalence point? Why or why not? Look at the volume used to titrate the polyprotic acid. What is the relationship between the volume used to reach the first equivalence point, and the second equivalence point? Why does that relationship exist? What would be your predicted value for pKa3?