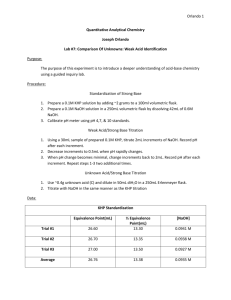
H3AsO4 + H2O
advertisement
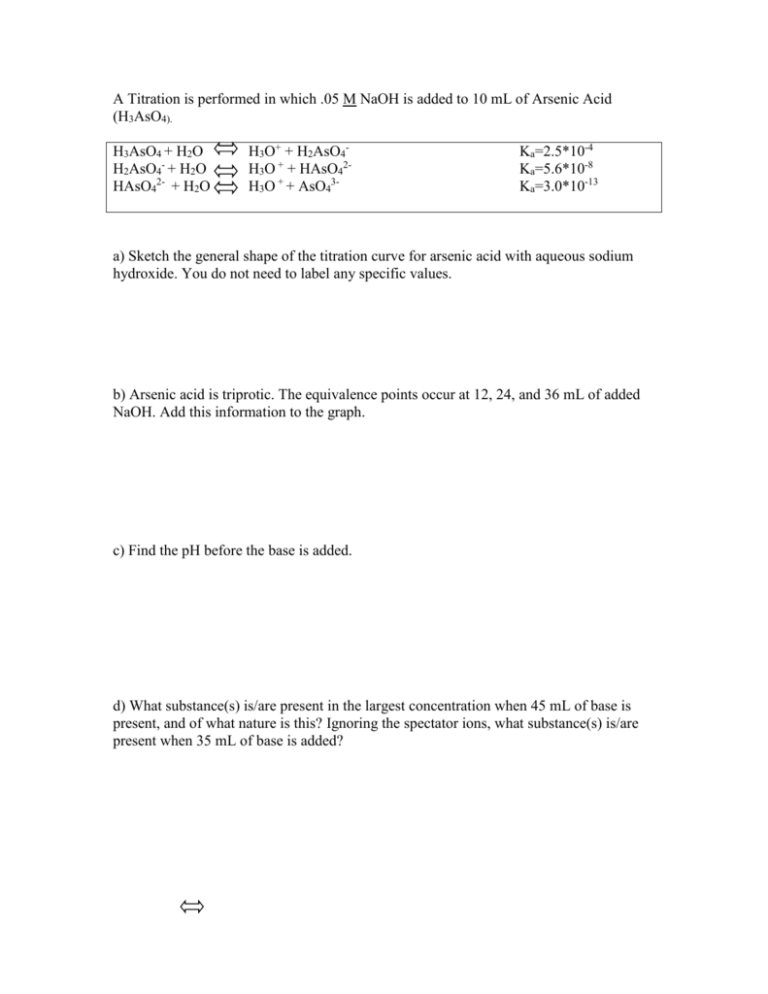
A Titration is performed in which .05 M NaOH is added to 10 mL of Arsenic Acid (H3AsO4). H3AsO4 + H2O H2AsO4- + H2O HAsO42- + H2O H3O+ + H2AsO4H3O + + HAsO42H3O + + AsO43- Ka=2.5*10-4 Ka=5.6*10-8 Ka=3.0*10-13 a) Sketch the general shape of the titration curve for arsenic acid with aqueous sodium hydroxide. You do not need to label any specific values. b) Arsenic acid is triprotic. The equivalence points occur at 12, 24, and 36 mL of added NaOH. Add this information to the graph. c) Find the pH before the base is added. d) What substance(s) is/are present in the largest concentration when 45 mL of base is present, and of what nature is this? Ignoring the spectator ions, what substance(s) is/are present when 35 mL of base is added? e) Calculate the pH of the solution when 7.2 mL of NaOH is added f) What is the pH at the second equivalence point? g) What is the pH of the solution when 30 mL of NaOH is added? h) You decide to empty the entire 50 mL bottle of NaOH into the flask. Find the pH. i) The titration experiment has been slightly altered. Pretend that the equivalence points and the volumes remain the same, but the concentration of the acid changes to .1 M. How is the pH at the second equivalence point different than the pH of the original experiment at the second equivalence point? j) When 12 mL of NaOH is added to the flask, what substance is present in the highest concentration? Of what nature is it? Calculate its concentration.