Weak Acid Titration Lab: Determine Concentration & Identity
Titration of a Weak Acid
Prepared by Thomas M. Moffett Jr., Plattsburgh State University, 2003
Objective: Determine the concentration and identity of an unknown acid by titration with a standard sodium hydroxide solution.
Background: Review acid/base titrations in your textbook.
A weak acid is a compound that partially ionizes in aqueous solution producing hydronium (H
3
O + ) ions. The general equation for the dissociation of any weak acid can be written as:
(aq) + H
3
(1) HA (aq) + H
2
O ( l ) ⇔ A O + (aq)
The addition of a strong base results in a neutralization reaction in which hydroxide ions
(OH ) react with hydronium to produce water:
H
3
O + (aq) + OH (aq) → 2 H
2
O ( l ) (2)
As hydronium is consumed in the neutralization reaction, the equilibrium in equation 1 is shifted to the right according to Le Chatelier’s Principle. The overall reaction for the neutralization process can be written as the sum of equations (1) and (2):
HA (aq) + OH (aq) → A (aq) + H
2
O ( l ) (3)
The equivalence point of the neutralization occurs when the moles of hydroxide are equal to the moles of hydronium. When a strong base neutralizes a weak acid, the equivalence point occurs at a pH greater than seven. This is due to the fact that the conjugate base of the weak acid (A ) will act as a weak base:
A (aq) + H
2
O ( l ) ⇔ HA (aq) + OH (aq) (4)
The concentration of the unknown solution can be determined by measuring the volume of titrant added to reach the equivalence point. At the equivalence point:
M
A
V
A
= M
B
V
B
(5)
Where M
A
is the molarity of the acid, M
B
is the molarity of the base and V
A and V
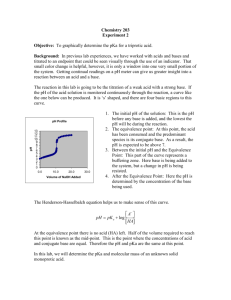
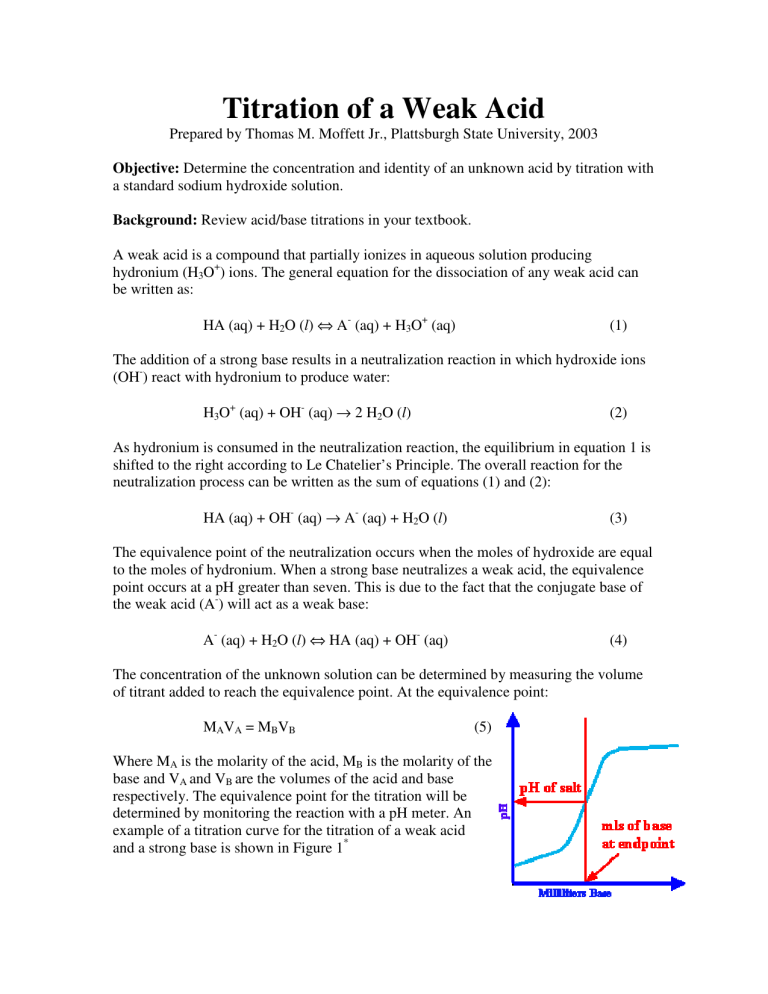
B are the volumes of the acid and base respectively. The equivalence point for the titration will be determined by monitoring the reaction with a pH meter. An example of a titration curve for the titration of a weak acid and a strong base is shown in Figure 1 *
The acid dissociation constant (K a
) can be determined in two ways. The first is graphically, the pK a
of the acid is equal to the pH at the midpoint of the buffer region.
This point can be found by using a little calculus (don’t worry, the computer will do it for you). The pK a
point is where the second derivative is equal to zero. Your instructor will go over how to find this from your data.
The second method involves using the pH at the equivalence point. Using equation 4 you can write an equilibrium expression for the weak base (note this is K b
). Use the pOH at the equivalence point and the concentration of the conjugate base to determine the value
.
K a of K b
( hint: think of all the acid being converted to base in equation 3 prior to the equilibrium in equation 4 – don’t forget to account for the volume change.
) The value of
can then be calculated using:
(6) K a
• K b
= K w
Using the value of the acid dissociation constant the identity of the acid can be
values): determined from the following list (you’ll have to look up the pK a
Name
Chlorous acid
Formic acid
Acetic acid
Formula
HClO
2
HCOOH
CH
3
COOH
Hypochlorous acid HOCl
Procedure: Safety goggles must be worn at all times in the laboratory.
1.) Obtain about 35 mL of the unknown acid solution in a clean dry Erlenmeyer flask. Also obtain about 70 mL of the sodium hydroxide solution in another clean dry Erlenmeyer flask (be sure to record the concentration).
2.) Rinse a 50 mL buret with two separate 5 mL portions of sodium hydroxide.
3.) Fill the buret with the NaOH, noting the starting volume.
4.) On the computer, start Logger Pro by double clicking on the icon on the desktop. Open the following file:
Chemistry With Computers\24a Acid-Base Titration
5.) Rinse a 25 mL pipette with a small portion of the acid solution.
6.) Pipette a 25.00 mL acid sample into a clean dry 150 mL beaker.
7.) Calibrate your pH sensor, follow the directions given by your instructor.
8.) In logger pro click on Collect (important: don’t click stop until the very end of the experiment). Insert the pH meter into the beaker with the acid, when the pH stabilizes click on Keep . A window will appear asking for you to enter a value, enter the total volume of NaOH which you have added (the first data point should be 0.00 – no NaOH added).
9.) Slowly add one to two mL of NaOH to the acid, stir, and record the pH by selecting Keep . Continue adding titrant in 1 to 2 mL increments until the pH reaches 5, at this point you should add titrant in approximately 0.2 to 0.4 mL intervals. When the pH curve flattens out again you can go back to adding 1 or
2 mL titrant samples, continue until the pH reaches 12.
10.) Click on Stop . Save an image of your graph for your report. You may also export your data as a text file.
Data Analysis
From your graph determine:
• the equivalence point (pH and volume of NaOH)
• the pK a
from the buffer region
Calculations:
• the concentration of the acid
• the pK a
•
from the equivalence point
%-error in your pK a
determinations
Questions:
1.) Write a balanced net-ionic reaction for the titration of your acid with NaOH.
2.) Explain why the pH at the equivalence point is not 7.00.
3.) Write a balanced net-ionic reaction for the titration of nitric acid with potassium hydroxide.
4.) The pOH of a solution is 3.78, determine pH, [H
3 solution acidic, basic, or neutral?
O + ], and [OH ]. Is the
Pre Lab Questions
1.) What are the Arrhenius definitions of an acid and a base?
2.) What are the Brønsted-Lowry definitions of an acid and a base?
3.) Write a reaction showing the autoionization of water.
4.) Hydrofluoric acid and sodium hydroxide react according to the following reaction:
HF (aq) + OH (aq) → F (aq) + H
2
O ( l )
How many milliliters of 0.250 M sodium hydroxide are needed to titrate 35.0 mL of
0.425 M hydrofluoric acid?