What is Chemistry?
advertisement

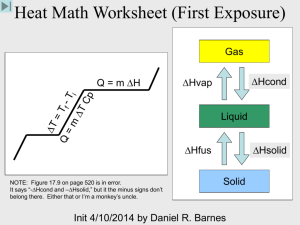
States of Matter Solid Lowest energy/heat Molecules barely moving Definite, uniform shape Example: ice States of Matter Liquid Medium energy/heat Molecules slowly moving Shape of container Example: water States of Matter Gas High energy Molecules moving rapidly and bouncing Fills its container Example: steam http://ds9.ssl.berkeley.edu/LWS_GEMS/2/part2.htm © UC Regents Phase Changes • Phase Change – when a during phase change! substance changes from • Examples: one state to another • Change of energy in “system.” • Solid liquid • Liquid gas • When energy is added, it • Solid gas is used to heat the substance, then it is used • Gas liquid to change the substance • Liquid solid into a different state. • Temp doesn’t change • Gas solid Phase Changes • Solid liquid melting • Liquid gas boiling, evaporation • Solid gas sublimation • Gas liquid condensation • Liquid solid freezing • Gas solid deposition Phase Changes GAS Condensation Sublimation Deposition Evaporation Melting SOLID Freezing LIQUID When a solid is heated… The temperature increases UNTIL It reaches it’s melting point THEN it turns to a liquid at that temperature The temperature will not change unless all matter is in the same state Ice will warm, then melt, the liquid will warm It takes energy for those molecules to change state! Heating/Cooling Curve Heat of Vaporization Heat of Fusion/Crystallization Energy is added as Heat Heating Curves Energy is added to the system at a constant rate Temperature increases at a constant rate UNLESS it is changing phase Phases The phase of a substance depends on: TEMPERATURE PRESSURE Phase diagram If you know T and P, you can figure out the state! The phase diagram is graph of pressure vs. temperature that shows conditions under which the phases of a substance exist http://itl.chem.ufl.edu/4411/2041/lec_f.html Triple Point: Unique temperature and pressure where all three phases exist…AT THE SAME TIME!!! Phase Diagrams for Water and CO2 *Atmospheric Pressure is 1.0 atm Phase Diagram Worksheet Try answering the questions about the mystery substance! Phase Changes A heating curve shows a substance’s change in temperature while adding heat energy Heating Curve ws Changes in Matter and Energy Matter cannot be created or destroyed. But it can be changed and when it does, that is how we get energy! Energy - capacity to do work or produce heat Energy is always involved in physical and chemical changes. Energy can take several forms: Heat, light, (sound, chemical, electrical) Measured in calories, Calories (kcal), and joules Law of Conservation of Energy: energy can be absorbed or released, but it cannot be created or destroyed through ordinary chemical reactions. Energy can be transferred. Kinetic and Potential Energy Kinetic energy: is the energy of motion. Potential Energy: energy of Position Stored energy (chemical bonds) Since energy is constant and cannot be created or destroyed …. Total Energy = KE + PE Temperature: kinetic energy of all particles within matter. There are times during phase changes when temperature does not change, but stays constant while the energy works to change the phase (ie: the heating curve of water) Heating/Cooling Curve Heat of Vaporization Heat of Fusion Energy is added as Heat Exothermic energy is released by the substance into the surroundings less PE, more KE, so temperature rises Ex: a match burning Endothermic energy is absorbed by the substance from the surroundings more PE, less KE, so temperature drops Ex: water freezing Energy Calorie (cal): the amount of energy (heat) required to raise the temperature of one gram of water by one Celcius degree Standard unit for energy is the joule (J) 1 cal = 4.184 J 60.1 cal x 4.184 J = 251 J 1 cal Specific Heat (s) : amount of energy required to change the temperature of one gram of a substance 1 oC Varies from one substance to another Heat always travels from high concentration to low concentration!! Heat lost = Heat gained Water has a specific heat = 1 cal/goC or 4.184 J/goC Water has the second highest specific heat capacity of all known substances. So it requires high amounts of heat energy to raise water temperature. water also has a high energy/heat requirement for evaporation SIRON = 0.449 J/goC Which would heat up faster, 5.00 grams of iron or 5.00 grams of water? Which would cool down faster, 5.00 grams of iron or 5.00 grams of water? Which is a better thermal conductor? Which is a better insulator? Q = s x m x DT Q = energy (heat) required (J) or (cal) s = specific heat capacity (J/goC) or (cal/goC) m = mass of the sample in grams DT = change in temperature in oC A 2.8 g sample of a pure metal requires 10.1 J of energy to change its temperature from 21 oC to 36 oC. What is the specific heat of the metal? s= Q m x DT = 10.1 J = 0.24 J/goC (2.8 g x 15oC)