Chemistry of pH Lab - Doral Academy Preparatory
advertisement

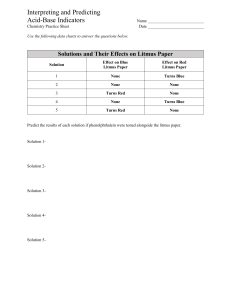
Chemistry of pH Lab Objectives of this Lab: • The student will be able to recognize an acid and a base on the PH scale. • The student will be able to read litmus and PH paper. 2 Lab Materials Used: • Common items –vinegar, hand sanitizer, fizzing antacid tablets, apple juice, baking soda, grapefruit juice, • Red Litmus Paper • Blue Litmus Paper 3 Procedures for this lab: • Label your plastic cups #'s 1-6 • Choose one solution and pour a small amount into plas tic cup #1. • Write the name of the solution in your data table. • Place a drop of the solution onto red and then blue lit mus paper. Record results • State whether each solution is an acid or a base. 4 Data: Check One Name of Substance PH Hypothesis Red to Blue Blue to Red 5 Check One No Change Acid Base Neutral 0-2 Most acidic lemon juice 3-4 apple, vinegar, tomato 5- banana 6- Milk 7-Neutral water 8- blood 8-9 baking soda 10-11 soap 11-12 ammonia 13-14 Most basic drain cleaner 6 How to Understand Litmus Paper Red Litmus Paper Results • Red paper turns blue (you are working with a base) • Red paper stays red (not a base) Blue Litmus Paper Results • Blue paper turns red (you are working with an acid) • Blue paper stays blue (not an acid) *If there is no color change, then you have a neutral substance. 7 Analysis Questions 1. Arrange the substances you tested on the pH scale by putting them in order from 0 to 14. 2. When the pH of a substance is taken, what is actually being done? 3. Stomach acid’s ph is between 2 and 3. Is this an acid or a base? 8 pH of Acids and Bases • Use the internet or other resources to fi nd the pH of each of the 6 substances. • Record the pH of each substance in the second column of your table. 9 How to write your conclusion • First paragraph: restate the objectives, materi als, and procedures used in this lab. • Second paragraph: discuss the results from the lab (mention all values on the data table). • Third paragraph: Describe what the pH scale is and what it is used for. Include as well the common items on the scale. • DUE NEXT CLASS NO EXCEPTIONS! 10