LAB: Acids and Bases
advertisement

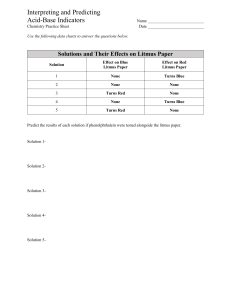
Name: ____________________________ Date: _____________ LAB: ACIDS AND BASES Indicators are special chemicals that can show whether a given substance is an acid, a base, or neither. Indicators usually react with an acid or base to form a slightly different chemical with a different color. The indicators we are using today are litmus paper (red and blue), and pH paper. Litmus paper turns blue in a base and red in an acid. One type of pH paper turns a different color at each of several pH values ranging from 1 to 14. Litmus paper can only be used to indicate the presence of a base or acid whereas the pH paper can tell you how strong the base or acid is. Acids will release hydrogen ions (H+) in solutions. Bases will release hydroxide ions (OH-). The pH scale ranges from 0 to 14 and measures the percent of hydrogen present. A pH of 7 is neutral (distilled water), a pH below 7 is acid (hydrochloric acid) and a pH above 7 is a base (sodium hydroxide). ***Before you begin the experiment, make a hypothesis about which substances will be acids and which ones will be bases. Do this by placing an A (acid), B (base) or N (neutral) in the appropriate column in the table. Procedure: Obtain the various indicator papers according to your teachers instructions Move about the room carefully dipping all three indicators into the substances. Record the resulting color or number. Keep the dry papers in ½ petri dish and the wet papers in the other ½ of the petri dish When finished, throw the wet papers away and return the dry papers. Rinse out the wet paper ½ of the petri dish, dry and return both to the designated area. Hypothesis 1. Substance Blue Litmus (resulting color) Red Litmus (resulting color) pH paper (numerical reading) Conclusion (acid, base or neutral) Blue litmus paper turns _______________ in an acid and stays ____________ in a base. 2. Red litmus paper turns _____________ in bases and stays ___________ in an acid. 3. Is litmus paper or pH paper more useful in determining the exact pH of a substance? Explain. 4. Which substance has the highest concentration of hydrogen ions (H+)? __________________________ Is this an acid or base? 5. Which substance has the highest concentration of hydroxide ions (OH-)? __________________________ Is this an acid or a base? 6. Which substances surprised you with their pH? Why? 7. Suppose you had two beakers of stomach acid in front of you. How could you investigate which of two antacids (brand X or brand Y) is more effective in neutralizing stomach acid? Use the back if needed.