Oxidation Numbers & Stock System Naming Worksheet
advertisement

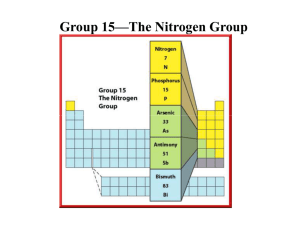
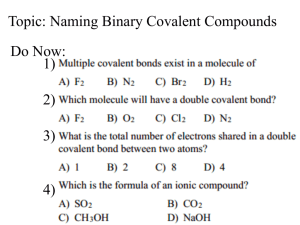
Name__________________________________________ Date ____________ Period _______ Assigning Oxidation Numbers Many nonmetals can have more than one oxidation number. These numbers can sometimes be used in the same manner as ionic charges to determine formulas. Assign oxidation numbers to each atom in the following compounds or ions: a. HF b. CS2 d. CLO2ˉ c. Na2O2 d. PI3 e. IO3ˉ The Stock System is actually based on oxidation numbers, and it can be used as an alternative to the prefix system for naming binary molecular compounds. PCl3 PCl5 N2O NO PbO2 Mo2O3 Prefix System phosphorus trichloride phosphorus pentachloride dinitrogen monoxide nitrogen monoxide lead dioxide dimolybdenum trioxide Stock System phosphorus(III) chloride phosphorus (IV) chloride nitrogen(I) oxide nitrogen(II) oxide lead (IV) oxide molybdenum(III) oxide Name each of the following binary molecular compounds according to the Stock System: a. CI4 b. SO3 c. As2S3 d. NCl3 e. CS2 f. KF g. PBr3