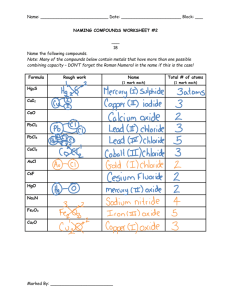
Ionic and Covalent Worksheet Name: _____________________________ Fill in the chart, as indicated. Name nitrogen dioxide Symbol NO2 Symbol CaO Name Calcium oxide calcium fluoride CaF2 SO2 Sulfur dioxide carbon monoxide CO CO2 Carbon Dioxide silicon tetraboride SiBr4 NaCl Sodium Chloride magnesium sulphide MgS CO Carbon Monoxide sodium phosphide Na3P MgS Magnesium Sulphide diboron trioxide B2O3 Cs3N Cesium Nitride CCl4 Carbon tetrachloride Complete the following table for the ionic compounds with more than one valence charge. Name titanium (III) iodide nickel (III) nitride cobalt (II) chloride manganese (VII) chloride Symbol TiI₃ NiN CoCl2 MnCl7 Iron (III) sulphide Symbol PbO FeN CuF Pb3N2 Name lead(II) oxide. Iron(III) nitride Copper (I) fluoride Lead (II) nitride SnS Fe₂S₃ Tin(II) sulfide FeP Iron(III) phosphide Sn3N2 Tin(II) Nitride PbF4 Lead(IV) fluoride SnO2 Tin(IV) oxide CuBr2 copper(II) bromide