Ideal Gas Law
advertisement

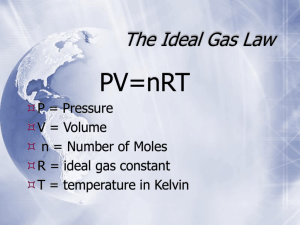
Gases Physical Properties Kinetic Molecular Theory Particles • • • • • in an ideal gas… have no volume have elastic collisions are in constant, random, straight-line motion don’t attract or repel each other average kinetic energy is directly proportional to the absolute temperature To change pressure you can: • Change temperature • Change volume • Change amount Characteristics of Gases Gases expand to fill any container • random motion, no attraction Gases are fluid (like liquids) • no attraction Gases have very low densities • no volume = lots of empty space Characteristics of Gases Gases can be compressed • no volume = lots of empty space Gases undergo diffusion & effusion • random motion State Changes Describing Gases Gases can be described by their: • Temperature •K • Pressure • atm • Volume •L • Number of molecules/moles • # Temperature Temperature: Every time temperature is used in a gas law equation it must be stated in Kelvin (an absolute temperature) ºF -459 ºC -273 K 0 C 59 F 32 32 212 0 100 273 373 K = ºC + 273 Pressure Pressure of a gas is the force of its particles exerted over a unit area (number of collisions against a container’s walls) force pressure area Which shoes create the most pressure? Pressure Pressure may have different units: torr, millimeters of mercury (mm Hg), atmospheres (atm), Pascals (Pa), or kilopascals (kPa). KEY EQUIVALENT UNITS • 760 torr • 760 mm Hg • 1 atm • 101,325 Pa • 101.325 kPa (kilopascal) • 14.7 psi STP STP Standard Temperature & Pressure 0°C 273 K -OR- 1 atm 101.325 kPa The volume of 1 mole of gas at STP is 22.4 L Pressure Problem 1 The average pressure in Denver, Colorado, is 0.830 atm. Express this in kPa. 0.830 atm 101.325 kPa= 84.1 1 atm kPa Pressure Problem 2 Convert a pressure of 1.75 atm to psi. 1.75 atm 14.7 psi 1 atm = 25.7 psi The Behavior of Gases The Gas Laws Boyle’s Law Investigated P the relationship between pressure and volume on a gas. He found that pressure and volume of a gas are inversely related • at constant mass & temp V Boyle’s Law As pressure increases Volume decreases Boyles LAW: P1V1 = k P V Boyle’s Law Gas Law Problems A gas occupies 100. mL at 150. kPa. Find its volume at 200. kPa. BOYLE’S LAW GIVEN: P V V1 = 100. mL P1 = 150. kPa V2 = ? P2 = 200. kPa WORK: P1V1 = P2V2 (150.kPa)(100.mL)=(200.kPa)V2 V2 = 75.0 mL Charles’ Law Jacques Charles investigated the relationship of temperature & volume in a gas. He found that the volume of a gas and the Kelvin temperature of a gas are directly related. V T V1 k T1 Charles’ Law As volume increases, temperature increases Charles V T Law: V1 k T1 Charles’ Law Gas Law Problems gas occupies 473 cm3 at 36°C. Find its volume at 94°C. A CHARLES’ LAW GIVEN: T V V1 = 473 cm3 T1 = 36°C = 309K V2 = ? T2 = 94°C = 367K WORK: V1T2 = V2T1 (473 cm3)(367 K)=V2(309 K) V2 = 562 cm3 Gas Law Problems Example: The volume of a sample of gas is 43.5 L at STP. What will the volume of the gas be if the temperature is increased to 35.4°C? CHARLES’ LAW GIVEN: T V V1 = 43.5 L T1 = 0°C = 273K V2 = ? T2= 35.4°C= 308.4K WORK: V1T2 = V2T1 (43.5 L)(308.4 K)=V2(273 K) V2 = 49.1 L Gay-Lussac’s Law Since temperature and volume are related, it would make sense that temperature and pressure are related. The equation for this will resemble Charle’s law: P1 k T1 P T Gay-Lussac’s Law The pressure and absolute temperature (K) of a gas are directly related • at constant mass & volume P1 k T1 P T Gas Law Problems A gas’ pressure is 765 torr at 23°C. At what temperature will the pressure be 560. torr? GAY-LUSSAC’S LAW GIVEN: P T WORK: P1 = 765 torr P1T2 = P2T1 T1 = 23°C = 296K (765 torr)T2 = (560. torr)(296K) P2 = 560. torr T2 = 217 K = -56°C T2 = ? Combined Gas Law P V PV PV = k T P 1V 1 P 2V 2 = T1 T2 P 1 V 1T 2 = P 2V 2 T 1 Gas Law Problems gas occupies 7.84 cm3 at 71.8 kPa & 25°C. Find its volume at STP. A COMBINED GAS LAW GIVEN: P T V WORK: V1 = 7.84 cm3 P1V1T2 = P2V2T1 P1 = 71.8 kPa (71.8 kPa)(7.84 cm3)(273 K) T1 = 25°C = 298 K =(101.325 kPa) V2 (298 K) V2 = ? P2 = 101.325 kPa V2 = 5.09 cm3 T2 = 273 K The First 4 Gas Laws The Gas Laws Table Gases II. Ideal Gas Law Ideal Gas Law and Gas Stoichiometry Ideal Gas Law Part 1 Avogadro’s Law The volume of a gas increases or reduces, as its number of moles is being increased or decreased. The volume of an enclosed gas is directly proportional to its number of moles. Avogadro’s Principle Equal volumes of gases contain equal numbers of moles • at constant temp & pressure • true for any gas • n = number of moles V1 k n1 V n Ideal Gas Law Merge the Combined Gas Law with Avogadro’s Principle: PV V =R k nT T n UNIVERSAL GAS CONSTANT R=0.08206 Latm/molK R=8.315 dm3kPa/molK Ideal Gas Law PV=nRT UNIVERSAL GAS CONSTANT R=0.08206 Latm/molK R=8.315 dm3kPa/molK Ideal Gas Law Problems Calculate the pressure in atmospheres of 0.412 mol of Heat 16°C & occupying 3.25 L. GIVEN: WORK: P = ? atm PV = nRT n = 0.412 mol P(3.25)=(0.412)(0.0821)(289) L mol Latm/molK K T = 16°C = 289 K V = 3.25 L P = 3.01 atm R = 0.08206Latm/molK Ideal Gas Law Problems Find the volume of 85 g of O2 at 25°C and 104.5 kPa. GIVEN: WORK: V=? 85 g 1 mol O2 = 2.7 mol n = 85 g = 2.7 mol 32.00 g O2 T = 25°C = 298 K PV = nRT P = 104.5 kPa (104.5)V=(2.7) (8.315) (298) kPa mol dm3kPa/molK K R = 8.315 dm3kPa/molK V = 64 dm3 Ideal Gas Law and Molar Mass The Ideal Gas Law can be used to calculate the mass or the molar mass of a gas sample if the mass of the sample is known. number of molesgas = substituting mass gas or ngas = mgas_ Molar Massgas MMgas this for ngas in PV = nRT P V = mgas__ R T MMgas yields Applications of Ideal Gas Law Calculate the grams of N2 present in a 0.60 L sample kept at 1.00 atm pressure and a temperature of 22.0oC. GIVEN: WORK: P = 1.00 atm T = 22°C = 295. K V = .600 L R = 0.08206 Latm/molK MM = 28.01 g mN2 = ? MM P V = mgas R T (28.01)(1.0)(.60)=m (.0821) (295) g/mol atm L Latm/molK K mN2 = .69 g of N2 Applications of Ideal Gas Law Can be used to calculate the molar mass of a gas and the density grams of a gas mass m n moles of a gas molar mass molar mass MM Substitute this into ideal gas law nRT m( RT ) P V MM (V ) And m/V = d in g/L, so dRT P MM or dRT MM P Applications of Ideal Gas Law The density of a gas was measured at 1.50 atm and 27°C and found to be 1.95 g/L. Calculate the molar mass of the gas. GIVEN: WORK: P = 1.50 atm MM = dRT/P T = 27°C = 300. K MM=(1.95)(0.08206)(300.)/1.50 g/L Latm/molK K atm d = 1.95 g/L R = 0.08206 Latm/molK MM = 32.0 g/mol MM = ? Applications of Ideal Gas Law Calculate the density of carbon dioxide gas at 25°C and 750. torr. GIVEN: WORK: 750 torr 1 atm = .987 atm d = ? g/L CO2 760 torr T = 25°C = 298 K MM = dRT/P →d = MM P/RT P = 750. torr = .987 atm d=(44.01 g/mol)(.987 atm) R = 0.08206 Latm/molK (0.08206 Latm/molK )(298K) MM = 44.01 g/mol d = 1.78 g/L CO2 Part 2 Gas Stoichiometry * Stoichiometry Steps Review * 1. Write a balanced equation. 2. Identify known & unknown. 3. Line up conversion factors. Mole ratio ratio -molesmoles moles •• Mole moles • Molar mass moles grams • Molarity moles liters soln • Molar volume - moles liters gas Core step in all stoichiometry problems!! 4. Check answer. Molar Volume at STP 1 mol of a gas=22.4 L at STP Standard Temperature & 0°C and 1 atm Pressure Molar Volume at STP LITERS OF GAS AT STP Molar Volume (22.4 L/mol) MASS IN GRAMS Molar Mass (g/mol) MOLES 6.02 1023 particles/mol NUMBER OF PARTICLES Gas Stoichiometry of one Gas Liters of another Gas: • Avogadro’s Principle • Coefficients give mole ratios and volume ratios Moles (or grams) of A Liters of B: • STP – use 22.4 L/mol • Non-STP – use ideal gas law & stoich Non-STP • Given liters of gas? start with ideal gas law • Looking for liters of gas? start with stoichiometry conversion Liters Gas Stoichiometry Problem – STP many grams of KClO3 are req’d to produce 9.00 L of O2 at STP? How 2KClO3 2KCl + 3O2 ?g 9.00 L 9.00 L O2 1 mol O2 2 mol KClO3 122.55 g KClO3 22.4 L O2 3 mol O2 1 mol KClO3 = 32.8 g KClO3 Gas Stoichiometry Problem – Non-STP What volume of CO2 forms from 5.25 g of CaCO3 at 103 kPa & 25ºC? CaCO3 5.25 g CaO Looking for liters: Start with stoich and calculate moles of CO2. 5.25 g 1 mol CaCO3 CaCO3 1 mol CO2 100.09g 1 mol CaCO3 CaCO3 + CO2 ?L non-STP P = 103 kPa V = ? NEXT n=? = 0.0525 mol CO2 R = 8.315 3kPa/molK dmthe Plug this into Ideal Gas Law for n to find liters T = 298K Gas Stoichiometry Problem – Non-STP What volume of CO2 forms from 5.25 g of CaCO3 at 103 kPa & 25ºC? GIVEN: WORK: P = 103 kPa V=? n = 0.0525 mol T = 25°C = 298 K R = 8.315 dm3kPa/molK PV = nRT (103 kPa)V =(0.0525mol)(8.315dm3kPa/molK) (298K) V = 1.26 dm3 CO2 Gas Stoichiometry Problem How many grams of Al2O3 are formed from 15.0 L of O2 at 97.3 kPa & 21°C? 4 Al + 3 O2 15.0 L non-STP 2 Al2O3 ?g GIVEN: WORK: P = 97.3 kPa V = 15.0 L n=? T = 21°C = 294 K R = 8.315 dm3kPa/molK PV = nRT (97.3 kPa) (15.0 L) = n (8.315dm3kPa/molK) (294K) Given liters: Start with Ideal Gas Law and calculate moles of O2. NEXT n = 0.597 mol O2 Gas Stoichiometry Problem How many grams of Al2O3 are formed from 15.0 L of O2 at 97.3 kPa & 21°C? 3 O2 15.0L Use stoich to convert moles of O to grams Al O non-STP 0.597 2 mol 101.96 g mol O2 Al2O3 Al2O3 4 Al 2 2 + 2 Al2O3 ?g 3 3 mol O2 1 mol Al2O3 = 40.6 g Al2O3 Ch. 14 - Gases III. Two More Laws Dalton’s Law & Graham’s Law A. Dalton’s Law The total pressure of a mixture of gases equals the sum of the partial pressures of the individual gases Ptotal = P1 + P2 + ...Pn A. Dalton’s Law A sample of air has a total pressure of 1.00 atm. The mixture contains only CO2, N2, and O2. If PCO2 = 0.12 atm and PN2 = 0.70, what is the partial pressure of O2? GIVEN: PO2 = ? Ptotal = 1.00 atm PCO2 = 0.12 atm PN2 = 0.70 atm WORK: Ptotal = PCO2 + PN2 + PO2 1.00 atm = 0.12 atm + .70 atm + PO2 PO2 = 0.18 atm A. Dalton’s Law Mole Fraction: the ratio of the number of moles of a given component in a mixture to the total number of moles in the mixture, represented by chi ( ) n1 nTOTAL n1 n1 n2 n3 ... Because n is directly proportional to P according to the ideal gas law, we can derive χA nA n TOTAL PA PTOTAL A. Dalton’s Law The mole fraction of nitrogen in the air is 0.7808. Calculate the partial pressure of N2 in air when the atmospheric pressure is 760. torr. GIVEN: χN2 = 0.7808 Ptotal = 760. torr PN2 = ? WORK: χN2 = PN2 /PTOTAL 0.7808 = (PN2)/(760. torr) PN2 = 593 torr A. Dalton’s Law •When H2 gas is collected by water displacement, the gas in the collection bottle is actually a mixture of H2 and water vapor •Water exerts a pressure known as water-vapor pressure •So, to determine total pressure of gas and water vapor inside a container, make total pressure inside bottle = atmosphere and use this formula: Ptotal = Pgas + PH2O A. Dalton’s Law Hydrogen gas is collected over water at 22.5°C. Find the pressure of the dry gas if the atmospheric pressure is 94.4 kPa. The total pressure in the collection bottle is equal to atmospheric pressure and is a mixture of H2 and water vapor. GIVEN: WORK: PH2 = ? Ptotal = PH2 + PH2O Ptotal = 94.4 kPa 94.4 kPa = PH2 + 2.72 kPa PH2O = 20.4 mm Hg P = 91.7 kPa H2 = 2.72 kPa Look up water-vapor pressure Sig Figs: Round to lowest for 22.5°C, convert to kPa number of decimal places. B. Graham’s Law Diffusion • Spreading of gas molecules throughout a container until evenly distributed Effusion • Passing of gas molecules through a tiny opening in a container B. Graham’s Law Speed of diffusion/effusion • Kinetic energy is determined by the temperature of the gas. • At the same temp & KE, heavier molecules move more slowly. Larger m smaller v KE = 2 ½mv B. Graham’s Law Graham’s Law • Rate of diffusion of a gas is inversely related to the square root of its molar mass. • The equation shows the ratio of Gas A’s speed to Gas B’s speed. rate A rate B MM B MM A B. Graham’s Law Determine the relative rate of diffusion for krypton and bromine. The first gas is “Gas A” and the second gas is “Gas B”. Relative rate means find the ratio “vA/vB”. vA vB v Kr v Br2 m Br2 m Kr mB mA 159.80 g/mol 1.381 83.80 g/mol Kr diffuses 1.381 times faster than Br2. B. Graham’s Law vA vB A molecule of oxygen gas has an average speed of 12.3 m/s at a given temp and pressure. What is the average speed of hydrogen molecules at the same conditions? mB mA vH 2 12.3 m/s 32.00 g/mol 2.02 g/mol vH 2 vH 2 vO2 mO2 mH 2 Put the gas with the unknown speed as “Gas A”. 12.3 m/s 3.980 vH2 49.0 m/s B. Graham’s Law An unknown gas diffuses 4.0 times faster than O2. Find its molar mass. The first gas is “Gas A” and the second gas is “Gas B”. The ratio “vA/vB” is 4.0. vA vB mB mA vA v O2 mO2 mA 32.00 g/mol 4.0 m AA 32.00 g/mol 16 mA Square both sides to get rid of the square root sign. 32.00 g/mol 2.0 g/mol mA 16 2