UNITY-1 Study: daclatasvir/asunaprevir/beclabuvir - HCV
advertisement
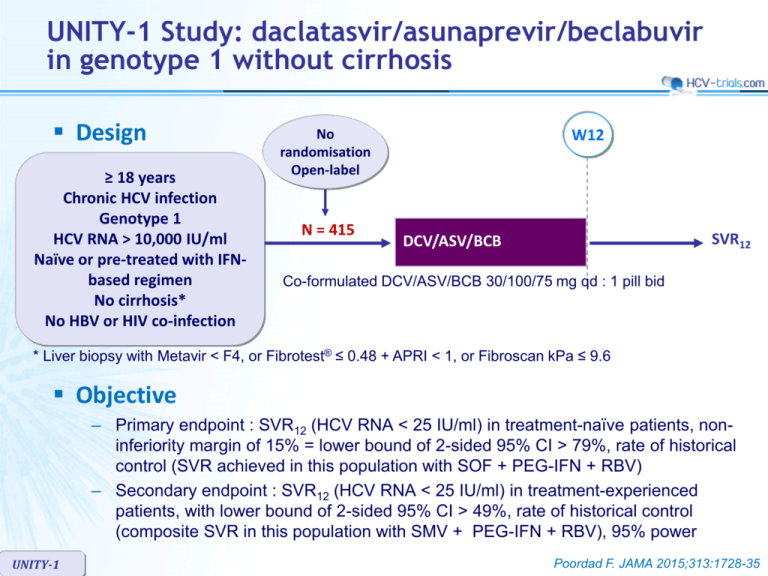
UNITY-1 Study: daclatasvir/asunaprevir/beclabuvir in genotype 1 without cirrhosis Design ≥ 18 years Chronic HCV infection Genotype 1 HCV RNA > 10,000 IU/ml Naïve or pre-treated with IFNbased regimen No cirrhosis* No HBV or HIV co-infection W12 No randomisation Open-label N = 415 SVR12 DCV/ASV/BCB Co-formulated DCV/ASV/BCB 30/100/75 mg qd : 1 pill bid * Liver biopsy with Metavir < F4, or Fibrotest® ≤ 0.48 + APRI < 1, or Fibroscan kPa ≤ 9.6 Objective – Primary endpoint : SVR12 (HCV RNA < 25 IU/ml) in treatment-naïve patients, noninferiority margin of 15% = lower bound of 2-sided 95% CI > 79%, rate of historical control (SVR achieved in this population with SOF + PEG-IFN + RBV) – Secondary endpoint : SVR12 (HCV RNA < 25 IU/ml) in treatment-experienced patients, with lower bound of 2-sided 95% CI > 49%, rate of historical control (composite SVR in this population with SMV + PEG-IFN + RBV), 95% power UNITY-1 Poordad F. JAMA 2015;313:1728-35 UNITY-1 Study: daclatasvir/asunaprevir/beclabuvir in genotype 1 without cirrhosis Baseline characteristics and patient disposition Treatmentnaïve N = 312 Treatmentexperienced N = 103 Median age, years 53.5 57 Female 44% 38% Race : white / black 87% / 11% 88% / 7% Genotype : 1a / 1b 73% / 27% 73% / 27% IL28B CC genotype 29% 16% HCV RNA > 800,000 IU/ml 78% 90% - 90.3% Prior IFN-based treatment, N (%) Relapse 37.9% Null response 24.3% Partial response 11.7% Interferon intolerant 6.8% Indeterminate 9.7% Discontinuation, N Lack of virologic response / adverse event / pregnancy UNITY-1 7 4 6/0/1 1/3/0 Poordad F. JAMA 2015;313:1728-35 UNITY-1 Study: daclatasvir/asunaprevir/beclabuvir in genotype 1 without cirrhosis SVR12 (HCV RNA < 25 IU/ml) , % (95% CI) All patients Naïve % 100 92* (89-95) 89.3* (83.4-95.3) Experienced 97.6 90 100 85.3 SVR12 comparable across subgroups : 75 – – – – 50 25 0 N 312 103 Non-response 3 2 Virologic breakthrough 6 2 Relapse 15 6 229 75 1a 83 Gender Age HCV RNA level IL28B genotype 28 1b * Superior to historical control UNITY-1 Poordad F. JAMA 2015;313:1728-35 UNITY-1 Study: daclatasvir/asunaprevir/beclabuvir in genotype 1 without cirrhosis Resistance analysis – Genotype 1a : 32 virologic failures • NS5A resistance-associated variants in 28/29 (most frequent : Q30) • NS3 RAVs in 25/26 (most frequent : R155) • NS5B RAVs in 12/28 (most frequent : P495) – Genotype 1b : 2 virologic failures – Baseline NS5A polymorphims (28, 30, 31, 93) associated with resistance to DCV • Genotype 1a : 34/102 (11%) : SVR12 in 25/34 (74%) • Genotype 1b : 17/106 (16%) : SVR12 in 17/17 (100%) – Baseline NS3 and NS5B variants did not affect SVR12 UNITY-1 Poordad F. JAMA 2015;313:1728-35 UNITY-1 Study: daclatasvir/asunaprevir/beclabuvir in genotype 1 without cirrhosis Adverse events and laboratory abnormalities, N N = 415 Discontinuation for adverse event 3 (0.7%) Serious adverse event 7 (1.7%) Adverse event in > 10% of patients Headache 25.8% Fatigue 16.6% Diarrhea 14.0% Nausea 13.5% Grade 3-4 laboratory abnormalities Hemoglobin < 9 g/dl UNITY-1 0 Lymphocytes < 0.5 x 109/l 1 (0.2%) Neutrophils < 0.75 x 109/l 2 (0.5%) ALT > 5 x ULN 19 (4.6%) AST > 5 x ULN 9 (2.2%) Lipase > 3 x ULN 16 (3.9%) Poordad F. JAMA 2015;313:1728-35 UNITY-1 Study: daclatasvir/asunaprevir/beclabuvir in genotype 1 without cirrhosis Summary – In this open-label, uncontrolled study, 12 weeks of treatment with a fixeddose combination of daclatasvir, asunaprevir and beclabuvir in HCV genotype 1-infected patients without cirrhosis was associated with high SVR12 • 92% in treatment-naive patients • 89% in patients previously treated for HCV infection • SVR12 rates were higher with genotype 1b than with genotype 1a in both the treatment-naive (98% vs 90%, respectively) and -experienced (100% vs 85%, respectively) cohorts – There were low rates of serious AEs and treatment discontinuations – All genotype 1b-infected patients with baseline NS5A polymorphisms achieved SVR12 while only 74% with genotype 1a had SVR12 – Resistance-associated variants at amino acids positions NS5A-Q30, NS3-R155K and NS5B-P495 were observed most frequently at viral breakthrough; NS5B variants were generally not observed in patients experiencing relapse UNITY-1 Poordad F. JAMA 2015;313:1728-35