e-configs 2013
advertisement

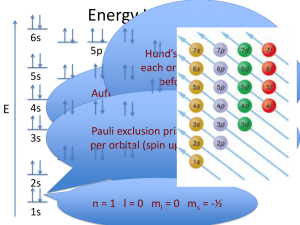
ELECTRON CONFIGURATION THE QUANTUM MECHANICAL MODEL The e- is found inside a blurry “electron cloud” An area where there is a chance of finding an electron. Only found with 90% accuracy in the area WHERE CAN WE FIND ELECTRONS? Each element has its own e- configuration for its ground state No charges We base them on where the LAST e- is placed IT’S LIKE AN ADDRESS… State – city – street – house # State is called the Principle Energy level City is called the sublevel Street is called the orbital House # is the e- spin direction PRINCIPLE ENERGY LEVELS We have 1-7 Highest occupied ground state energy level = PT period # Main energy levels Bohr found SUBLEVELS Each energy level has increasing number of sublevels Level 1 has 1 sublevel Level 2 has 2 sublevels Level 3 has 3 sublevels And so on…. Though 7 major levels…only 4 main sublevels are needed to describe existing atoms SUBLEVEL Sublevels are distinguished by the shape of orbitals in them There are currently four of them s p d f ORBITALS In each sublevel there are specific orbitals An Orbital is a 3-D region in space an e- can be found Does not have “hard and fast” boundaries See next slide Each orbital can hold only 2 e Mathematically found through wave function WHAT’S GOING ON… S - SUBLEVEL 1 orbital in this sublevel Sphere shape Total 2 e- possible Same shape in each level…just bigger Found in ALL energy levels p - SUBLEVEL 3 orbitals in this sublevel Dumbbell shaped Total 6 e Found in Energy Level 2 or Higher Node between each half of each orbital Intermediate area between high probability areas d - SUBLEVEL 5 orbitals in this sublevel Clover shape Total 10 possible e Found in Energy Level 3 or higher. f - SUBLEVEL 7 orbitals in this sublevel Total 14 e- possible Complex shape Found in Energy Level 4 or higher ENERGY - SUBLEVEL Sublevels 1 2 s s, p 3 4 5 s, p, d s, p, d, f s, p, d, f, g 6 7 s, p, d, f, g, h s, p, d, f, g, h, i We will only commonly use s, p, d, and f!! HOW WE WRITE WHERE E- ARE State – city – street – house # State is called the principle E level City is called the sublevel Street is called the orbital House # is the e- spin direction 5 32 e18 e8 e2 e- 4 3 2 1 f d p s nucleus Increasing energy 7s 6s 5s 7p 6p 5p 4p 4s 3p 3s 2p 2s 1s 6d 5d 4d 3d 5f 4f 7s 6s 5s 7p 6p 6d 5f 5d 5p 4f 4d 4p 3d 4s 3p 3s 2p 2s 1s 1s 2 = 1s ELECTRON CONFIGURATION RULES The way electrons are arranged in atoms. Aufbau principle- electrons enter the lowest energy first. Must fully fill before move to next This causes difficulties because of the overlap of orbitals of different energies. RULES CONTINUED Pauli Exclusion Principle- no 2 e- can have same set of 4 quantum # at most 2 electrons per orbital - different spins!! Hund’s Rule- “up, up, up before down, down, down” All orbitals need to be filled w/one “up” spin (positive--clockwise) before any in the sublevel is filled with a “down” spin (negative-counterclockwise) DRAWING ORBITAL NOTATION A box is used to represent each orbital. Arrows are used to represent each electron. **remember opposite spins. Example: the orbital notation for carbon is: 1s 2s 2p ORBITAL NOTATION Lets try a few: Orbital Notation for O O = 8 e1s 2s 2p Orbital Notation for Cl Cl 1s = 17e- 2s 2p 3s 3p Electron Configuration # of electrons 1s Principle Energy Level 2 sublevel Interpret the following Electron Configuration 4 electrons 4 3p 3rd Energy Level p sublevel WHEN DOING A CONFIGURATION… Use the total number of e Slowly place e- in order till run out of e Example B 5e- 2 in 1s2…3 left over Next 2 can go is 2s2 …1 left over Last one goes in 2p, but since only 1 left it is 2p1 Final configuration 1s2 2s2 2p1 First TRY A FEW K Cl Fe Pb ANSWERS K 1s2 2s2 2p6 3s2 3p6 4s1 Cl 1s2 2s2 2p6 3s2 3p5 Fe 1s2 2s2 2p6 3s2 3p6 4s2 3d6 Pb 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 6s2 5d10 6p2 5s2 4d10 5p6 TONIGHT’S HOMEWORK Write out the e- configuration for elements Hydrogen through and including Yttrium DO NOW: Box all things written with the HIGHEST PRINCIPLE energy level for each configuration of your homework Ex: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 Circle the LAST THING WRITTEN for each configuration of your homework Ex: 1s2 2s2 2p6 3s2 3p6 4s2 3d6 OUTERLEVEL (SHELL) CONFIGURATION Everything is the outside/highest PRINCPLE ENERGY LEVEL Max is 8 e- total 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 EXAMPLES Ga Ra Mn Se Am Br EXAMPLES Ga- 4s24p1 Ra- 7s2 Mn- 4s2 Se- 4s24p4 Am- 7s2 Br- 4s24p5 SUBLEVEL (SHELL) CONFIGURATION The last thing written for the e- configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d6 Every element has its own ground level subshell configuration unique to itself EXAMPLES F Ca Al Pa Mo Xe EXAMPLES F- 2p5 Ca- 4s2 Al- 3p1 Pa- 5f3 Mo- 4d4 Xe- 5p6 NOBLE GAS CONFIGURATIONS In order to save time and your hand when writing out electron configurations, one may use the Noble Gas Notation. A noble gas symbol is used in place of a long list of electron configurations. Example: Ar: 1s2 2s2 2p6 3s2 3p6 = [Ar] Noble Gas Shorthand Configuration for Ca Ca: [Ar] 4s2 EXAMPLES – NOBLE GAS CONFIGURATIONS Sr W Hg Cl CHARGED ATOMS Can do e- configurations for ions, but must make sure to note that that is what you are doing! Same way, just use the number of e- in the ion involved