Chemistry Unit 14 Practice Test
advertisement

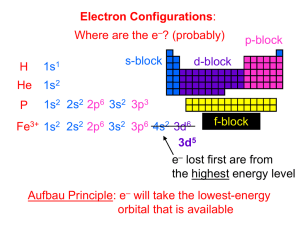
Chemistry Unit 4 Practice Test Electronic Structure in Atoms No work shown = no credit. Please write legibly. Numerical answers must be rounded to the appropriate number of significant figures or decimal points and must contain the appropriate unit. 1. Write the full electron configuration for the following elements. Each item is worth 2 points (10 points). (A) oxygen (element 8) ___1s2 2s2 2p4______________________ (B) silicon (element 14) ___1s2 2s2 2p6 3s2 3p2________________ (C) titanium (element 22) ___1s2 2s2 2p6 3s2 3p6 4s2 3d2__________ (D) nickel (element 28) ___1s2 2s2 2p6 3s2 3p6 4s2 3d8__________ (E) bromine (element 35) ___1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5______ 2. Write the noble gas core configuration for the following elements. Each item is worth 2 points (10 points). (A) zirconium (element 40) _______[Kr] 5s2 4d2__________________ (B) tin (element 50) _______[Kr] 5s2 4d10 5p2______________ (C) neodymium (element 60) _______[Xe] 6s2 4f4__________________ (D) ytterbium (element 70) _______[Xe] 6s2 4f14_________________ (E) mercury (element 80) _______[Xe] 6s2 4f14 5d10______________ 3. Given the electron configuration, draw orbital diagrams for the following elements. Each item is worth 5 points (25 points). (A) hydrogen (1s1) 1s (B) boron (1s2 2s2 2p1) 1s 2s (C) neon (1s2 2s2 2p6) 2p 1s 2s 2p (D) potassium (1s2 2s2 2p6 3s2 3p6 4s1) 1s 2s 2p 3s (E) vanadium (1s2 2s2 2p6 3s2 3p6 4s2 3d3) 1s 2s 2p 3s 3p 4s 3p 4s 3d 4. Identify the number of valence electrons in each of the following elements. Each item is worth 1 point (10 points). (A) Al (element 13) __3__ (F) Li (element 3) __1__ (B) Ar (element 18) __8__ (G) Mg (element 12) __2__ (C) F (element 9) __7__ (H) N (element 7) __5__ (D) H (element 1) __1__ (I) Se (element 34) __6__ (E) He (element 2) __2__ (J) Si (element 14) __4__ 5. Draw electron dot diagrams for each of the following elements. Each item is worth 2 points (10 points). (A) Ba (element 56) Ba Br (B) Br (element 35) (C) K (element 19) K Ne (D) Ne (element 10) (E) S (element 16) S 6. TRUE or FALSE: The higher the frequency of electromagnetic radiation (light), the more energy it carries. Explain your answer. (You will not get full credit just for writing “True” or “False”) (10 points) TRUE. The energy carried by light is directly proportional to its frequency. 7. Define the following term: emission spectrum. (10 points) Emission spectrum – wavelengths of light given off by an element when it is excited. Every element has a unique emission spectrum. 8. TRUE or FALSE: When an electron absorbs one quantum of energy, it jumps to a lower energy level. Explain your answer. (You will not get full credit just for writing “True” or “False”) (10 points) FALSE. When electrons are excited by a quantum of energy, they jump to a higher energy level, not a lower one. 9. Briefly describe how a flame test can be used to identify some metals in compounds. (5 points) Some metals give a characteristic color to a flame (copper = green, sodium = orange, etc). By observing the color of a flame that is passing over a salt, one can determine what metal the salt contains.