Alkynes
advertisement

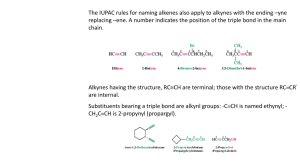
Alkynes • Alkynes - hydrocarbons with carboncarbon triple bonds • Alkadiynes - hydrocarbons with two carbon-carbon triple bonds • Alkenynes - hydrocarbons with both double and triple bonds Nomenclature of Alkynes • 1. Find the longest continuous chain of carbon atoms containing the multiple bonds and name it using –yne, -diyne, or –en-yne. • 2. Number the chain so the multiple bonds have the lowest possible number – **if numbering from either end gives the same number for the multiple bonds, then the double bond gets preference over the triple bond (the double bond gets the lower number) • 3. Name, number and alphabetize the constituent groups. Alkyne Nomenclature Examples • 1. • 2. • 3. • 4. • 5. Reactions of Alkynes • Alkynes undergo similar reactions to the alkenes (addition reactions) • 1. Hydrogenation – addition of H2 • -the usual product is an alkane, BUT…with the use of a special catalyst known as Lindlar’s catalyst (Pd), it is possible to stop the reaction at the alkene • -this is especially useful for preparing cis alkenes, since both hydrogens add to the triple bond from the same side • Hydrogenation Examples • 1. Hydrogenate 2-butyne with Lindlar’s reagent. • 2. Hydrogenate 2-butyne with Pt or Ni. • 2. Halogenation – addition of Cl2 or Br2 – this is a trans addition – Ex.; Brominate ethyne. • 3. Hydrohalogenation - addition of HI, HBr, or HCl • - follows Markovnikoff’s rule – H attaches to the carbon with the greater number of hydrogens • Ex.; React propyne with HCl. • 4. Hydration - addition of H-OH • - follows Markovnikoff’s rule • - catalyst is a mixture of dilute sulfuric acid (H2SO4) and mercuric sulfate (HgSO4) • -product is an unstable enol, which rearranges itself to form an aldehyde or a ketone • -enols have a double bond (-ene) and an alcohol (-ol) on the same carbon atom • Hydration examples. • 1. Hydrate propyne • 2. Hydrate ethyne Preparation of Alkynes • Dehydrohalogenation of a Dihalide - removal of HCl or HBr (two of them) using KOH and ethanol (C2H5OH) • - most commonly used to convert an alkene to an alkyne in a synthesis sequence • Ex. 1. Dehydrohalogenate 2,3dibromobutane. • Ex. 2. Dehydrohalogenate 2,2dibromobutane. • Ex. 3. Synthesize propyne from propene.