1-1 Properties of Alkenes and alkynes
advertisement

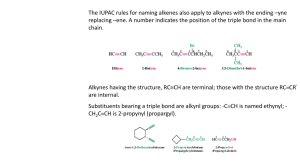
Properties of Alkenes (at least 1 double bond) and alkynes (at least 1 triple bond) Physical properties dependent on The size of the molecule The number of double or triple bonds present As the size increases - State moves from gas to liquid to solid just like alkanes As the number of double or triple bonds increases the Melting point Boiling point Density decreases Melting, boiling and density differences here can be explained by the fact that larger molecules with double and triple bonds have kinks in their shape causing poor packing. Not miscible in water Chemical properties dependent on the fact that alkenes and alkynes are not fully saturated. They are unsaturated. Most reactions of these molecules are faster than in alkanes Alkenes and Alkynes Chemists often group these two families together as unsaturated hydrocarbons. Do you remember what alkanes were called if alkenes and alkynes are unsaturated? Alkenes have the general formula CnH2n, and end in “ene” Alkynes have the general formula CnH2n-2, and end in “yne”. What is the general formula for alkanes? The position of the multiple bonds is indicated by a number in front of the name. Treat double and triple bonds as branches. Greek prefixes are used before the ending if there are more than one double or triple bond (i.e. 2 doubles bonds = a “diene”). Formula CH2=CH2 CH2=CHCH3 CH2= CH CH2CH3 CH3CH=CHCH3 CH2= CH CH=CH2 Name Ethene Propene 1-butene 2-butene 1,3-butadiene Structure 2 carbon atoms 3 carbon atoms Double bond at carbon 1 Double bond at carbon 2 Double bonds at carbon 1 and 3. Notice the number of hydrogens around each carbon. Remember carbon can only form FOUR bonds. A double bond counts as two and a triple bond counts as three. The same rules apply for naming alkynes you just use the “yne” ending instead of “ene.” Notice that we put an “a” after But in the last example…just so that it can be pronounced. ? Why don’t we need to indicate the position of the double bond in examples 1 and 2? ? Examples: Name the following hydrocarbons. 1. C3H4 3. 2. C5H10 4. Draw structural, condensed and line formulas for the following alkanes. 1. 2-butene 2. 3-octyne What are the general formulas for alkanes, alkenes and alkynes?